Monday Poster Session
Category: IBD
P2215 - Efficacy and Safety of Omilancor in a Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Crohn’s Disease
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- AL
Andrew Leber, PhD
NIMML Institute
Blacksburg, VA
Presenting Author(s)
Andrew Leber, PhD, Raquel Hontecillas, PhD, Nuria Tubau Juni, PhD, Josep Bassaganya-Riera, PhD
NIMML Institute, Blacksburg, VA
Introduction: Omilancor is an oral, gut-restricted, first-in-class LANCL2 agonist for ulcerative colitis (UC) and Crohn’s disease (CD). Through LANCL2 activation, omilancor locally increases the suppressive capacity of regulatory immune cells, including regulatory CD4+ T cells (Tregs), and restores metabolic deficiencies in the intestinal mucosa. Once daily omilancor was well tolerated up to doses of 7500 mg/day for one week in a Phase I study in healthy volunteers.
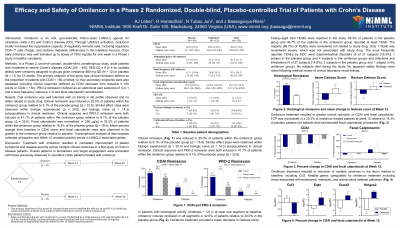
Methods: In a Phase 2, proof-of-concept, double-blind, parallel-group study, adult patients with moderate to severe Crohn’s disease (CDAI 220 – 450; SES-CD ≥ 6 [≥ 4 for isolated ileitis]) were randomly assigned to groups given omilancor 880 mg QD (N = 12) or placebo (N = 11) for 12 weeks. The primary endpoint of the study was clinical remission defined as the proportion of patients with CDAI < 150 at Week 12. Key secondary endpoints were also assessed including clinical response (defined as CDAI decrease from baseline ≥ 100 points or CDAI < 150), PRO-2 remission (defined as an abdominal pain subscore of 0 or 1 and a stool frequency subscore ≤ 3) and fecal calprotectin normalization.
Results: Oral omilancor was well tolerated with no trends in AE profile observed and no SAEs related to study drug. Clinical remission was induced in 25.0% of patients within the omilancor group relative to 9.1% of the placebo group (Δ = 15.9). Similar effect sizes were observed within biologic experienced (Δ = 20.0) and biologic naïve (Δ = 14.3) subpopulations in clinical remission. Clinical response and PRO-2 remission were both induced in 41.7% of patients within the omilancor group relative to 9.1% of the placebo group (Δ = 32.6). Fecal calprotectin was normalized (< 250 µg/g) in 33.3% of patients within the omilancor group relative to 14.3% of the placebo group (Δ = 19.0). Mean percent change from baseline in CDAI score and fecal calprotectin were also observed to be greater in the omilancor group relative to placebo. Transcriptional analysis of ileal biopsies collected at baseline and Week 12 revealed positive trends in LANCL2 associated genes.
Discussion: Treatment with omilancor resulted in consistent improvement of patient symptoms and disease severity across multiple clinical outcomes in a first study of Crohn’s disease patients. Overall patterns in biomarkers and target engagement were consistent with those previously observed in ulcerative colitis patients treated with omilancor.
Disclosures:
Andrew Leber, PhD, Raquel Hontecillas, PhD, Nuria Tubau Juni, PhD, Josep Bassaganya-Riera, PhD. P2215 - Efficacy and Safety of Omilancor in a Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Crohn’s Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
NIMML Institute, Blacksburg, VA
Introduction: Omilancor is an oral, gut-restricted, first-in-class LANCL2 agonist for ulcerative colitis (UC) and Crohn’s disease (CD). Through LANCL2 activation, omilancor locally increases the suppressive capacity of regulatory immune cells, including regulatory CD4+ T cells (Tregs), and restores metabolic deficiencies in the intestinal mucosa. Once daily omilancor was well tolerated up to doses of 7500 mg/day for one week in a Phase I study in healthy volunteers.
Methods: In a Phase 2, proof-of-concept, double-blind, parallel-group study, adult patients with moderate to severe Crohn’s disease (CDAI 220 – 450; SES-CD ≥ 6 [≥ 4 for isolated ileitis]) were randomly assigned to groups given omilancor 880 mg QD (N = 12) or placebo (N = 11) for 12 weeks. The primary endpoint of the study was clinical remission defined as the proportion of patients with CDAI < 150 at Week 12. Key secondary endpoints were also assessed including clinical response (defined as CDAI decrease from baseline ≥ 100 points or CDAI < 150), PRO-2 remission (defined as an abdominal pain subscore of 0 or 1 and a stool frequency subscore ≤ 3) and fecal calprotectin normalization.
Results: Oral omilancor was well tolerated with no trends in AE profile observed and no SAEs related to study drug. Clinical remission was induced in 25.0% of patients within the omilancor group relative to 9.1% of the placebo group (Δ = 15.9). Similar effect sizes were observed within biologic experienced (Δ = 20.0) and biologic naïve (Δ = 14.3) subpopulations in clinical remission. Clinical response and PRO-2 remission were both induced in 41.7% of patients within the omilancor group relative to 9.1% of the placebo group (Δ = 32.6). Fecal calprotectin was normalized (< 250 µg/g) in 33.3% of patients within the omilancor group relative to 14.3% of the placebo group (Δ = 19.0). Mean percent change from baseline in CDAI score and fecal calprotectin were also observed to be greater in the omilancor group relative to placebo. Transcriptional analysis of ileal biopsies collected at baseline and Week 12 revealed positive trends in LANCL2 associated genes.
Discussion: Treatment with omilancor resulted in consistent improvement of patient symptoms and disease severity across multiple clinical outcomes in a first study of Crohn’s disease patients. Overall patterns in biomarkers and target engagement were consistent with those previously observed in ulcerative colitis patients treated with omilancor.
Disclosures:
Andrew Leber indicated no relevant financial relationships.
Raquel Hontecillas indicated no relevant financial relationships.
Nuria Tubau Juni indicated no relevant financial relationships.
Josep Bassaganya-Riera: Landos Biopharma – Employee, Stock-publicly held company(excluding mutual/index funds).
Andrew Leber, PhD, Raquel Hontecillas, PhD, Nuria Tubau Juni, PhD, Josep Bassaganya-Riera, PhD. P2215 - Efficacy and Safety of Omilancor in a Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Crohn’s Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
