Monday Poster Session
Category: IBD
P2231 - Induction of Remission in TNF-Alpha Inhibitor Refractory Acute Severe Ulcerative Colitis With Upadacitinib: A Case Report
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Colin P. Martyn, MD
Indiana University
Newburgh, IN
Presenting Author(s)
Colin P. Martyn, MD1, Konstantin Boroda, MD2
1Indiana University, Newburgh, IN; 2Digestive Care Center, Newburgh, IN
Introduction: Upadacitinib is a novel oral small-molecule selective inhibitor of the Janus kinase (JAK1) enzyme that recently received FDA approval for TNF-alpha inhibitor refractory moderate to severe ulcerative colitis (UC) and Crohn’s disease. It has garnered recognition as a promising therapeutic option with the added convenience of once-daily oral dosing without a serious side effect profile. We present a case of a 24-year-old man with TNF-alpha inhibitor refractory UC complicated by recurrent C. difficile positivity who rapidly improved upon induction with Upadacitinib.
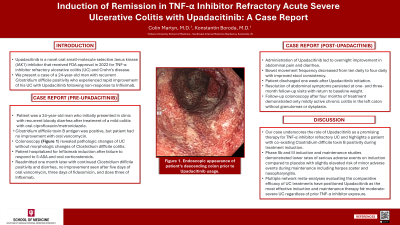
Case Description/Methods: A 24-year-old man without past medical history presented to our clinic for recurrent bloody diarrhea following a mild colitis 8 months prior treated with oral ciprofloxacin/metronidazole. C. difficile toxin B antigen was positive and diagnostic colonoscopy was performed after oral vancomycin failed to improve his symptoms. Pathological changes of UC were observed without morphological changes of C. difficile. Subsequent UC treatment with various 5-aminosalicylates and oral corticosteroids failed to produce lasting symptom relief, leading to initial hospitalization for Infliximab induction with readmission 1 month later. Induction with Upadacitinib 45 mg was administered after 5 days of oral vancomycin, 1 day of fidaxomicin, and a third dose of Infliximab failed to improve his symptoms. Overnight improvement was seen after one dose of Upadacitinib. He was discharged 1 week later on a regular diet without abdominal complaints. C. difficile toxin B antigen remained positive. Colonoscopy after 4 weeks of treatment showed only mildly active chronic left sided colitis.
Discussion: Our case underscores the role of upadacitinib as a promising therapy for TNF-alpha inhibitor refractory UC and highlights a patient with co-existing C. difficile toxin B positivity during treatment induction. Upadacitinib has been shown to be a comparably safe treatment option as phase IIb and III induction and maintenance studies demonstrated lower rates of serious adverse events on induction compared to placebo with a slightly elevated risk of minor adverse events during maintenance including herpes zoster and nasopharyngitis. While further head-to-head studies are warranted, multiple network meta-analyses evaluating the comparative efficacy of UC treatments have positioned upadacitinib as the most effective induction and maintenance treatment for moderate-severe UC regardless of prior TNF-alpha inhibitor exposure.
Disclosures:
Colin P. Martyn, MD1, Konstantin Boroda, MD2. P2231 - Induction of Remission in TNF-Alpha Inhibitor Refractory Acute Severe Ulcerative Colitis With Upadacitinib: A Case Report, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Indiana University, Newburgh, IN; 2Digestive Care Center, Newburgh, IN
Introduction: Upadacitinib is a novel oral small-molecule selective inhibitor of the Janus kinase (JAK1) enzyme that recently received FDA approval for TNF-alpha inhibitor refractory moderate to severe ulcerative colitis (UC) and Crohn’s disease. It has garnered recognition as a promising therapeutic option with the added convenience of once-daily oral dosing without a serious side effect profile. We present a case of a 24-year-old man with TNF-alpha inhibitor refractory UC complicated by recurrent C. difficile positivity who rapidly improved upon induction with Upadacitinib.
Case Description/Methods: A 24-year-old man without past medical history presented to our clinic for recurrent bloody diarrhea following a mild colitis 8 months prior treated with oral ciprofloxacin/metronidazole. C. difficile toxin B antigen was positive and diagnostic colonoscopy was performed after oral vancomycin failed to improve his symptoms. Pathological changes of UC were observed without morphological changes of C. difficile. Subsequent UC treatment with various 5-aminosalicylates and oral corticosteroids failed to produce lasting symptom relief, leading to initial hospitalization for Infliximab induction with readmission 1 month later. Induction with Upadacitinib 45 mg was administered after 5 days of oral vancomycin, 1 day of fidaxomicin, and a third dose of Infliximab failed to improve his symptoms. Overnight improvement was seen after one dose of Upadacitinib. He was discharged 1 week later on a regular diet without abdominal complaints. C. difficile toxin B antigen remained positive. Colonoscopy after 4 weeks of treatment showed only mildly active chronic left sided colitis.
Discussion: Our case underscores the role of upadacitinib as a promising therapy for TNF-alpha inhibitor refractory UC and highlights a patient with co-existing C. difficile toxin B positivity during treatment induction. Upadacitinib has been shown to be a comparably safe treatment option as phase IIb and III induction and maintenance studies demonstrated lower rates of serious adverse events on induction compared to placebo with a slightly elevated risk of minor adverse events during maintenance including herpes zoster and nasopharyngitis. While further head-to-head studies are warranted, multiple network meta-analyses evaluating the comparative efficacy of UC treatments have positioned upadacitinib as the most effective induction and maintenance treatment for moderate-severe UC regardless of prior TNF-alpha inhibitor exposure.
Disclosures:
Colin Martyn indicated no relevant financial relationships.
Konstantin Boroda indicated no relevant financial relationships.
Colin P. Martyn, MD1, Konstantin Boroda, MD2. P2231 - Induction of Remission in TNF-Alpha Inhibitor Refractory Acute Severe Ulcerative Colitis With Upadacitinib: A Case Report, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
