Monday Poster Session
Category: IBD
P2252 - The New “It Drug” for Ulcerative Colitis: Considerations for Use in High-Risk Patients
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- TL
Trina Liz Lewis, DO
Medical City Fort Worth
Bedford, TX
Presenting Author(s)
Mira Ibrahim, BS1, Trina Liz. Lewis, DO2, Long Hoang, DO3
1University of North Texas Health Science Center, Fort Worth, TX; 2Medical City Fort Worth, Bedford, TX; 3Medical City Fort Worth, Fort Worth, TX
Introduction: Ulcerative Colitis (UC) is an inflammatory bowel disease that effects the large intestine. Tofacitinib, a JAK inhibitor, is an oral medication approved for the management of moderate to severe UC. Although reports of bowel perforation exist in those taking tofacitinib for rheumatoid arthritis (thought to be associated with concomitant NSAID use), there are no such reports in those taking it for UC. Listed under important safety information for tofacitinib—GI perforation remains a risk and should be used with caution in those who are at an increased risk for this complication. In this report, we describe a case of bowel perforation in a patient with a history of diverticulitis who was placed on tofacitinib for management of UC.
Case Description/Methods: Our patient is an 82-year-old male with history of diverticulitis complicated by bowel perforation requiring a partial colectomy in 2008 who presented for evaluation of severe abdominal pain eight days after initiation of tofacitinib for newly diagnosed UC. One month prior to this, he had been hospitalized for lower abdominal pain associated with bloody stools for three months duration. CT abdomen at that time demonstrated diffuse circumferential rectal wall thickening with surrounding pericolonic inflammatory changes. He was diagnosed with a perirectal abscess and discharged home with antibiotics. He underwent a colonoscopy ten days later which revealed diffuse continuous ulceration with associated proctitis and spontaneous bleeding in the rectosigmoid colon. Biopsy findings were consistent with UC. Our patient was started on high dose extended release tofacitinib at 22mg qd along with high dose oral prednisone for management of UC.
The primary inquiry of this case study was to identify what factors were implicated in the patient’s bowel perforation. We theorize that the patient’s history of diverticulitis may have put him at increased risk of perforation following the initiation of tofacitinib. Although the role of JAK inhibition resulting in GI perforation remains unknown, this needs to be considered prior to initiation of tofacitinib in high-risk populations, such as those with diverticulitis.
Discussion: This case highlights the rare yet life-threatening risk of bowel perforation in patients on tofacitinib who have a history of diverticulitis. Although the first of its kind as an oral medication for the management of UC, the side effects and risks the medication poses are to be taken into careful consideration.

Disclosures:
Mira Ibrahim, BS1, Trina Liz. Lewis, DO2, Long Hoang, DO3. P2252 - The New “It Drug” for Ulcerative Colitis: Considerations for Use in High-Risk Patients, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of North Texas Health Science Center, Fort Worth, TX; 2Medical City Fort Worth, Bedford, TX; 3Medical City Fort Worth, Fort Worth, TX
Introduction: Ulcerative Colitis (UC) is an inflammatory bowel disease that effects the large intestine. Tofacitinib, a JAK inhibitor, is an oral medication approved for the management of moderate to severe UC. Although reports of bowel perforation exist in those taking tofacitinib for rheumatoid arthritis (thought to be associated with concomitant NSAID use), there are no such reports in those taking it for UC. Listed under important safety information for tofacitinib—GI perforation remains a risk and should be used with caution in those who are at an increased risk for this complication. In this report, we describe a case of bowel perforation in a patient with a history of diverticulitis who was placed on tofacitinib for management of UC.
Case Description/Methods: Our patient is an 82-year-old male with history of diverticulitis complicated by bowel perforation requiring a partial colectomy in 2008 who presented for evaluation of severe abdominal pain eight days after initiation of tofacitinib for newly diagnosed UC. One month prior to this, he had been hospitalized for lower abdominal pain associated with bloody stools for three months duration. CT abdomen at that time demonstrated diffuse circumferential rectal wall thickening with surrounding pericolonic inflammatory changes. He was diagnosed with a perirectal abscess and discharged home with antibiotics. He underwent a colonoscopy ten days later which revealed diffuse continuous ulceration with associated proctitis and spontaneous bleeding in the rectosigmoid colon. Biopsy findings were consistent with UC. Our patient was started on high dose extended release tofacitinib at 22mg qd along with high dose oral prednisone for management of UC.
The primary inquiry of this case study was to identify what factors were implicated in the patient’s bowel perforation. We theorize that the patient’s history of diverticulitis may have put him at increased risk of perforation following the initiation of tofacitinib. Although the role of JAK inhibition resulting in GI perforation remains unknown, this needs to be considered prior to initiation of tofacitinib in high-risk populations, such as those with diverticulitis.
Discussion: This case highlights the rare yet life-threatening risk of bowel perforation in patients on tofacitinib who have a history of diverticulitis. Although the first of its kind as an oral medication for the management of UC, the side effects and risks the medication poses are to be taken into careful consideration.

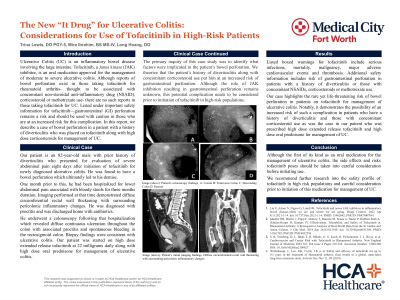
Figure: A: Cecum
B: Transverse Colon
C: Descending Colon
D: Descending Colon
E: Rectum
B: Transverse Colon
C: Descending Colon
D: Descending Colon
E: Rectum
Disclosures:
Mira Ibrahim indicated no relevant financial relationships.
Trina Lewis indicated no relevant financial relationships.
Long Hoang indicated no relevant financial relationships.
Mira Ibrahim, BS1, Trina Liz. Lewis, DO2, Long Hoang, DO3. P2252 - The New “It Drug” for Ulcerative Colitis: Considerations for Use in High-Risk Patients, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
