Monday Poster Session
Category: IBD
P2254 - Therapeutic Drug Monitoring of Vedolizumab in Management of Immune Checkpoint Inhibitor-Mediated Colitis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Anusha Thomas, MD
University of Texas MD Anderson Cancer Center
Houston, Texas
Presenting Author(s)
Award: Presidential Poster Award
Ninoska N. Silva, PA-C, MPAS, Yinghong Wang, MD, Anusha Thomas, MD
University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Immune-checkpoint inhibitors (ICI) have revolutionized cancer management of advanced malignancies. The unmitigated immune response triggered by checkpoint blockade can predispose to immune related adverse events (irAE). Moderate to severe immune-checkpoint inhibitor (ICI) diarrhea/colitis (IMC), one such irAE, is treated with selective immunosuppressive therapy (SIT) such as infliximab or vedolizumab (VDZ). Given the gut selective action of VDZ against the A4B7 integrins, systemic complications are minimal, making it ideal in the setting of underlying malignancy and pre-existing immunosuppression from cancer therapies. IMC refractory to VDZ is treated with experimental therapies such as fecal microbiota transplantation (FMT), IL12/23 blockade therapy etc. We explore the utility of therapeutic drug monitoring in patients on VDZ for IMC.
Case Description/Methods: We reviewed patients seen at our ambulatory center on ICI for varied stage IV cancers who received VDZ for IMC. Baseline stool inflammatory markers and endoscopic severity are reported. Response to treatment was assessed after induction phase of VDZ (300 mg IV administered at 0, 2 and 6 weeks) in terms of clinical improvement, fecal calprotectin trend, drug levels of VDZ and presence of VDZ antibodies obtained 2 weeks post 3rd infusion (Table1).
Our sample (n=5) comprised Caucasian men and women with a mean age of 69 years. Three had stage 4 GU related cancers and two had head & Neck/Lung cancers. Four received anti-PD-1 monotherapy. All patients had >/= CTCAE grade 2 diarrhea. Only one patient had ulcerative inflammation. VDZ levels were therapeutic in all 5 patients. All but one patient attained clinical remission. The primary non responder was steroid dependent with severe pan ulcerative colonic inflammation on colonoscopy. Repeat colonoscopy showed progressive inflammation with clinical remission attained after FMT.
Discussion: Ensuring adequate drug levels with therapeutic drug monitoring and trending inflammatory biomarkers viz. calprotectin may serve as non invasive methods to assess for treatment response to biologics in IMC. However, presence of high risk endoscopic features of IMC characterized by ulcers deeper than 2 mm, larger than 1 cm, and the presence of extensive colonic involvement should prompt providers to anticipate a much harder to treat, aggressive course of IMC despite therapeutic drug levels in biologic naive patients. Larger studies would aid in providing additional insight into adequate management.

Disclosures:
Ninoska N. Silva, PA-C, MPAS, Yinghong Wang, MD, Anusha Thomas, MD. P2254 - Therapeutic Drug Monitoring of Vedolizumab in Management of Immune Checkpoint Inhibitor-Mediated Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Ninoska N. Silva, PA-C, MPAS, Yinghong Wang, MD, Anusha Thomas, MD
University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Immune-checkpoint inhibitors (ICI) have revolutionized cancer management of advanced malignancies. The unmitigated immune response triggered by checkpoint blockade can predispose to immune related adverse events (irAE). Moderate to severe immune-checkpoint inhibitor (ICI) diarrhea/colitis (IMC), one such irAE, is treated with selective immunosuppressive therapy (SIT) such as infliximab or vedolizumab (VDZ). Given the gut selective action of VDZ against the A4B7 integrins, systemic complications are minimal, making it ideal in the setting of underlying malignancy and pre-existing immunosuppression from cancer therapies. IMC refractory to VDZ is treated with experimental therapies such as fecal microbiota transplantation (FMT), IL12/23 blockade therapy etc. We explore the utility of therapeutic drug monitoring in patients on VDZ for IMC.
Case Description/Methods: We reviewed patients seen at our ambulatory center on ICI for varied stage IV cancers who received VDZ for IMC. Baseline stool inflammatory markers and endoscopic severity are reported. Response to treatment was assessed after induction phase of VDZ (300 mg IV administered at 0, 2 and 6 weeks) in terms of clinical improvement, fecal calprotectin trend, drug levels of VDZ and presence of VDZ antibodies obtained 2 weeks post 3rd infusion (Table1).
Our sample (n=5) comprised Caucasian men and women with a mean age of 69 years. Three had stage 4 GU related cancers and two had head & Neck/Lung cancers. Four received anti-PD-1 monotherapy. All patients had >/= CTCAE grade 2 diarrhea. Only one patient had ulcerative inflammation. VDZ levels were therapeutic in all 5 patients. All but one patient attained clinical remission. The primary non responder was steroid dependent with severe pan ulcerative colonic inflammation on colonoscopy. Repeat colonoscopy showed progressive inflammation with clinical remission attained after FMT.
Discussion: Ensuring adequate drug levels with therapeutic drug monitoring and trending inflammatory biomarkers viz. calprotectin may serve as non invasive methods to assess for treatment response to biologics in IMC. However, presence of high risk endoscopic features of IMC characterized by ulcers deeper than 2 mm, larger than 1 cm, and the presence of extensive colonic involvement should prompt providers to anticipate a much harder to treat, aggressive course of IMC despite therapeutic drug levels in biologic naive patients. Larger studies would aid in providing additional insight into adequate management.

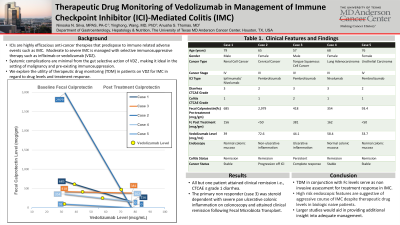
Figure: Figure 1. Trend of Fecal Calprotectin Pre-and Post Induction Phase of Vedolizumab.
Trend plotted per patient. Single non-responsive patient (case 3) depicted in orange, to be compared to responsive patients depicted in blue.
Yellow circle represents Vedolizumab level which is plotted along Fecal Calprotectin trend line for each corresponding patient.
Images show pre-treatment lower endoscopic findings.
Image A. Case 1 - Normal appearing colon mucosa with stool present (descending colon).
Image B. Case 2 - Significant edema and inflammation (ascending colon).
Image C. Case 3 - Diffuse severely congested, erythematous, eroded and hemorrhagic colon mucosa (ascending colon).
Image D. Case 4 - Normal appearing colon mucosa (ascending colon).
Image E. Case 5 - Normal appearing colon mucosa (descending colon).
Trend plotted per patient. Single non-responsive patient (case 3) depicted in orange, to be compared to responsive patients depicted in blue.
Yellow circle represents Vedolizumab level which is plotted along Fecal Calprotectin trend line for each corresponding patient.
Images show pre-treatment lower endoscopic findings.
Image A. Case 1 - Normal appearing colon mucosa with stool present (descending colon).
Image B. Case 2 - Significant edema and inflammation (ascending colon).
Image C. Case 3 - Diffuse severely congested, erythematous, eroded and hemorrhagic colon mucosa (ascending colon).
Image D. Case 4 - Normal appearing colon mucosa (ascending colon).
Image E. Case 5 - Normal appearing colon mucosa (descending colon).
Disclosures:
Ninoska Silva indicated no relevant financial relationships.
Yinghong Wang: ilyapharma – Consultant. IOTA – Consultant. Janssen – Consultant. MabQuest – Advisory Committee/Board Member. Sorriso – Consultant. Tillotts – Consultant.
Anusha Thomas indicated no relevant financial relationships.
Ninoska N. Silva, PA-C, MPAS, Yinghong Wang, MD, Anusha Thomas, MD. P2254 - Therapeutic Drug Monitoring of Vedolizumab in Management of Immune Checkpoint Inhibitor-Mediated Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

