Monday Poster Session
Category: Interventional Endoscopy
P2271 - Evaluation of the Performance and Safety of a Polysaccharide-Based Submucosal Injection Solution in EMR and ESD
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Sartajdeep Kahlon, MD
University of California Davis Medical
Sacramento, CA
Presenting Author(s)
Sartajdeep Kahlon, MD1, Manan Jhaveri, MD2, Shiro Urayama, MD2
1University of California Davis Medical, Sacramento, CA; 2University of California Davis Medical Center, Sacramento, CA
Introduction: In Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD), submucosal lifting agents optimize safety and successful removal of target lesions. Recently, crystalloid-oil emulsion solutions (COES) have been used in place of saline injection but have potential for peri-procedural bleeding. Starch-based polysaccharide solutions (SPS), which in powder form are used as an effective clotting agent in GI tract bleeding, are now available as an alternative lifting agent. This study compares SPS to COES injected cases to assess outcomes in patients undergoing EMR and ESD.
Methods: This was a retrospective study of patients who underwent EMR or ESD and received submucosal injection of either a SPS or a COES at a single academic hospital from 03/2021 to 03/2023. 46 patients were included in the COES group and 56 in the SPS group. Patient clinical data, procedure data, outcomes and pathology reports were extracted from chart review. Intraprocedural bleeding was defined as bleeding during a procedure requiring hemostatic intervention. Adverse events were instances of perforation or post-procedure GI bleed. Proportions of outcomes were compared and significance was determined using the Fisher exact test.
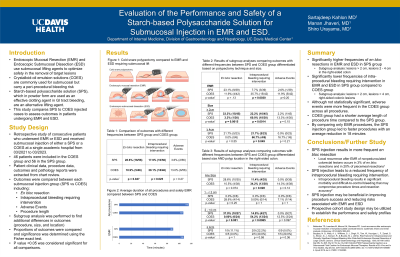
Results: Successful resection was achieved in all 102 patients included for evaluation. Average ages were 66 and 68 in the SPS and COES group, respectively. SPS group average lesion size was 2.8 cm while COES group average lesion size was 2.2 cm. En bloc resection was achieved 17.7% more frequently in patients receiving SPS submucosal injection (p = 0.047). Intraprocedural bleeding occurred significantly 21.2% more frequently in patients who received COES injection during EMR or ESD (p = 0.025). Adverse events were 9.4% more frequent in the COES group (p = 0.27). All episodes of perforation occurred in the COES group. In patients who underwent EMR for polypectomy, intraprocedural bleeding occurred significantly more in the COES group (p = 0.0030). SPS resulted in less intraprocedural bleeding for polypectomy of polyps ≥ 2cm (p = 0.0014) and polyps ≥ 4cm (p = 0.049). In the right-sided colon, large polyps (≥2 cm) had 39.4% more intraprocedural bleeding with COES injection.
Discussion: SPS injection may be beneficial over COES to limit intraprocedural bleeding. Adverse events occurred at a similar rate but perforation only occurred with COES injection. Thus, SPS should be considered for further study as a useful submucosal injection agent especially in resection of larger polyps.
Disclosures:
Sartajdeep Kahlon, MD1, Manan Jhaveri, MD2, Shiro Urayama, MD2. P2271 - Evaluation of the Performance and Safety of a Polysaccharide-Based Submucosal Injection Solution in EMR and ESD, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of California Davis Medical, Sacramento, CA; 2University of California Davis Medical Center, Sacramento, CA
Introduction: In Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD), submucosal lifting agents optimize safety and successful removal of target lesions. Recently, crystalloid-oil emulsion solutions (COES) have been used in place of saline injection but have potential for peri-procedural bleeding. Starch-based polysaccharide solutions (SPS), which in powder form are used as an effective clotting agent in GI tract bleeding, are now available as an alternative lifting agent. This study compares SPS to COES injected cases to assess outcomes in patients undergoing EMR and ESD.
Methods: This was a retrospective study of patients who underwent EMR or ESD and received submucosal injection of either a SPS or a COES at a single academic hospital from 03/2021 to 03/2023. 46 patients were included in the COES group and 56 in the SPS group. Patient clinical data, procedure data, outcomes and pathology reports were extracted from chart review. Intraprocedural bleeding was defined as bleeding during a procedure requiring hemostatic intervention. Adverse events were instances of perforation or post-procedure GI bleed. Proportions of outcomes were compared and significance was determined using the Fisher exact test.
Results: Successful resection was achieved in all 102 patients included for evaluation. Average ages were 66 and 68 in the SPS and COES group, respectively. SPS group average lesion size was 2.8 cm while COES group average lesion size was 2.2 cm. En bloc resection was achieved 17.7% more frequently in patients receiving SPS submucosal injection (p = 0.047). Intraprocedural bleeding occurred significantly 21.2% more frequently in patients who received COES injection during EMR or ESD (p = 0.025). Adverse events were 9.4% more frequent in the COES group (p = 0.27). All episodes of perforation occurred in the COES group. In patients who underwent EMR for polypectomy, intraprocedural bleeding occurred significantly more in the COES group (p = 0.0030). SPS resulted in less intraprocedural bleeding for polypectomy of polyps ≥ 2cm (p = 0.0014) and polyps ≥ 4cm (p = 0.049). In the right-sided colon, large polyps (≥2 cm) had 39.4% more intraprocedural bleeding with COES injection.
Discussion: SPS injection may be beneficial over COES to limit intraprocedural bleeding. Adverse events occurred at a similar rate but perforation only occurred with COES injection. Thus, SPS should be considered for further study as a useful submucosal injection agent especially in resection of larger polyps.
Disclosures:
Sartajdeep Kahlon indicated no relevant financial relationships.
Manan Jhaveri indicated no relevant financial relationships.
Shiro Urayama indicated no relevant financial relationships.
Sartajdeep Kahlon, MD1, Manan Jhaveri, MD2, Shiro Urayama, MD2. P2271 - Evaluation of the Performance and Safety of a Polysaccharide-Based Submucosal Injection Solution in EMR and ESD, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
