Monday Poster Session
Category: Interventional Endoscopy
P2282 - Adverse Events Associated With Different Endoscopic Ultrasound Fine Needle Biopsy (EUS-FNB) Needles: A MAUDE Database Analysis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Sobaan Taj, MD
Hackensack Meridian Jersey Shore University Medical Center
Neptune, NJ
Presenting Author(s)
Sobaan Taj, MD1, Daryl Ramai, MD, MSc2, Joseph Heaton, MD3, Daniel Mozell, MD4, Aimen Farooq, MD5, Saurabh Chandan, MD6, Antonio Facciorusso, MD7
1Hackensack Meridian Jersey Shore University Medical Center, Neptune, NJ; 2University of Utah, Salt Lake City, UT; 3Jersey Shore University Medical Center, Neptune, NJ; 4Elmhurst Hospital, Woodbridge, NJ; 5AdventHealth, Orlando, FL; 6Creighton University School of Medicine, Omaha, NE; 7University of Foggia, Foggia, Italy, Neptune, NJ
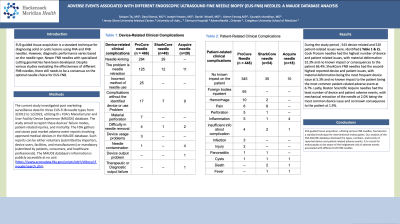
Introduction: EUS-guided tissue acquisition is a standard technique for diagnosing solid or cystic lesions using FNA and FNB needles. However, diagnostic performance varies based on the needle type. Newer FNB needles with specialized cutting geometries have been developed. Despite various studies evaluating the effectiveness of different FNB needles, there still needs to be a consensus on the optimal needle choice for EUS-FNB.
Methods: The current study investigated post marketing surveillance data for three EUS-FNB needle types from 3/2012 to 12/2022, utilizing the FDA's Manufacturer and User Facility Device Experience (MAUDE) database. The study aimed to report these devices' failure modes, patient-related injuries, and mortality. The FDA gathers and stores post market adverse event reports involving approved medical devices in the MAUDE database. Such reports can be either voluntary (submitted by importers, device users, facilities, and manufacturers) or mandatory (submitted by patients, consumers, and healthcare professionals). The MAUDE database's information is publicly accessible at no cost (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm).
Results: During the study period 498 events were identified, with Cook Procore needles accounting for the majority at 85.5%. Additionally, 543 device-related and 520 patient-related issues were identified. Cook Procore needles had the highest number of device and patient related issues, with material deformation 52.3% and no known impact or consequences to the patient 66.4%. SharkCore FNB needles had the second-highest reported device and patient issues, with material deformation being the most frequent device issue at 5.3% and no known impact to the patient being the most common patient-related adverse event at 6.7%. Lastly, Boston Scientific Acquire needles had the least number of device and patient adverse events, with mechanical retraction of the needle at 2.0% being the most common device issue and no known consequence to the patient at 1.9%.
Discussion: EUS-guided tissue acquisition, utilizing various FNB needles, has become a standard technique for interventional endoscopists. Our analysis of the FDA MAUDE database disclosed the types, numbers, and trends of reported device and patient-related adverse events. It is crucial for endoscopists to be aware of the heightened risk of adverse events associated with different EUS FNB needles.
Disclosures:
Sobaan Taj, MD1, Daryl Ramai, MD, MSc2, Joseph Heaton, MD3, Daniel Mozell, MD4, Aimen Farooq, MD5, Saurabh Chandan, MD6, Antonio Facciorusso, MD7. P2282 - Adverse Events Associated With Different Endoscopic Ultrasound Fine Needle Biopsy (EUS-FNB) Needles: A MAUDE Database Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Hackensack Meridian Jersey Shore University Medical Center, Neptune, NJ; 2University of Utah, Salt Lake City, UT; 3Jersey Shore University Medical Center, Neptune, NJ; 4Elmhurst Hospital, Woodbridge, NJ; 5AdventHealth, Orlando, FL; 6Creighton University School of Medicine, Omaha, NE; 7University of Foggia, Foggia, Italy, Neptune, NJ
Introduction: EUS-guided tissue acquisition is a standard technique for diagnosing solid or cystic lesions using FNA and FNB needles. However, diagnostic performance varies based on the needle type. Newer FNB needles with specialized cutting geometries have been developed. Despite various studies evaluating the effectiveness of different FNB needles, there still needs to be a consensus on the optimal needle choice for EUS-FNB.
Methods: The current study investigated post marketing surveillance data for three EUS-FNB needle types from 3/2012 to 12/2022, utilizing the FDA's Manufacturer and User Facility Device Experience (MAUDE) database. The study aimed to report these devices' failure modes, patient-related injuries, and mortality. The FDA gathers and stores post market adverse event reports involving approved medical devices in the MAUDE database. Such reports can be either voluntary (submitted by importers, device users, facilities, and manufacturers) or mandatory (submitted by patients, consumers, and healthcare professionals). The MAUDE database's information is publicly accessible at no cost (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm).
Results: During the study period 498 events were identified, with Cook Procore needles accounting for the majority at 85.5%. Additionally, 543 device-related and 520 patient-related issues were identified. Cook Procore needles had the highest number of device and patient related issues, with material deformation 52.3% and no known impact or consequences to the patient 66.4%. SharkCore FNB needles had the second-highest reported device and patient issues, with material deformation being the most frequent device issue at 5.3% and no known impact to the patient being the most common patient-related adverse event at 6.7%. Lastly, Boston Scientific Acquire needles had the least number of device and patient adverse events, with mechanical retraction of the needle at 2.0% being the most common device issue and no known consequence to the patient at 1.9%.
Discussion: EUS-guided tissue acquisition, utilizing various FNB needles, has become a standard technique for interventional endoscopists. Our analysis of the FDA MAUDE database disclosed the types, numbers, and trends of reported device and patient-related adverse events. It is crucial for endoscopists to be aware of the heightened risk of adverse events associated with different EUS FNB needles.
Disclosures:
Sobaan Taj indicated no relevant financial relationships.
Daryl Ramai indicated no relevant financial relationships.
Joseph Heaton indicated no relevant financial relationships.
Daniel Mozell indicated no relevant financial relationships.
Aimen Farooq indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Antonio Facciorusso indicated no relevant financial relationships.
Sobaan Taj, MD1, Daryl Ramai, MD, MSc2, Joseph Heaton, MD3, Daniel Mozell, MD4, Aimen Farooq, MD5, Saurabh Chandan, MD6, Antonio Facciorusso, MD7. P2282 - Adverse Events Associated With Different Endoscopic Ultrasound Fine Needle Biopsy (EUS-FNB) Needles: A MAUDE Database Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
