Monday Poster Session
Category: Liver
P2400 - Efficacy of L-Ornithine L-Aspartate as a Stand-Alone Treatment for Hepatic Encephalopathy in Patients With Cirrhosis - A Systematic Review and Meta-Analysis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall

Mythili Menon Pathiyil, MD
Saint Vincent Hospital
Worcester, MA
Presenting Author(s)
Mythili Menon Pathiyil, MD1, Arvind Kumar, MD1, Rutwik Pradeep Sharma, MD2, Sanket Basida, MD3, Jassimran Singh, MD1, Sajjadh MJ Ali, MD1, Shaji Sebastian, MD4
1Saint Vincent Hospital, Worcester, MA; 2Rochester Unity, Rochester, NY; 3University of Missouri at Columbia, Columbia, MO; 4Hull University Teaching Hospitals, Hull, England, United Kingdom
Introduction: There remains inconsistent data about the efficacy of L-Ornithine L-Aspartate in managing hepatic encephalopathy as a stand-alone treatment in patients with cirrhosis of various etiologies. This systematic review and meta-analysis was conducted to determine LOLA’s effect compared to supportive management in improving mental status in patients with cirrhosis.
Methods: We searched Pubmed, EMBASE, and Google Scholar for randomized controlled trials evaluating L-Ornithine L-Aspartate as a stand-alone therapy and comparing it to placebo on patients with cirrhosis presenting with different grades of hepatic encephalopathy. We excluded studies that did not provide relevant data, studies that had compared the efficacy of LOLA as an add-on therapy, and unpublished trials. All analyses were done in R version 4.1.1 using the meta and metafor packages. The random effects model was used with an inverse variance approach for pooled prevalence and M-H method for calculating relative risk.
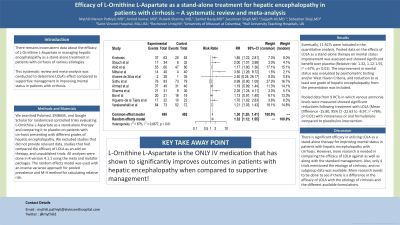
Results: Eventually, 11 RCTs were included in the quantitative analysis. Pooled data on the effects of LOLA as a stand-alone therapy on mental status improvement was assessed and showed significant benefit over placebo (Relative risk: 1.32, 1.12-1.55, I2 = 67%, p< 0.01). The improvement in mental status was evaluated by psychometric testing and/or West Haven Criteria, and reduction to at least one grade of hepatic encephalopathy from the presentation was included.
Pooled data from 9 RCTs in which venous ammonia levels were measured showed significant reductions following treatment with LOLA (Mean Difference -15.80, 95% CI -22.63 to -8.97, I2 =76%, p< 0.01) with intravenous or oral formulations compared to placebo/no intervention.
Discussion: There is significant efficacy in utilizing LOLA as a stand-alone therapy for improving mental status in patients with hepatic encephalopathy with cirrhosis. However, more research is needed in comparing the efficacy of LOLA against as well as along with the standard management. Also, only 4 trials mentioned the etiology of cirrhosis, and no subgroup data was available. More research needs to be done to see if there is a difference in the efficacy of LOLA with the etiology of cirrhosis and the different available formulations.

Disclosures:
Mythili Menon Pathiyil, MD1, Arvind Kumar, MD1, Rutwik Pradeep Sharma, MD2, Sanket Basida, MD3, Jassimran Singh, MD1, Sajjadh MJ Ali, MD1, Shaji Sebastian, MD4. P2400 - Efficacy of L-Ornithine L-Aspartate as a Stand-Alone Treatment for Hepatic Encephalopathy in Patients With Cirrhosis - A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Saint Vincent Hospital, Worcester, MA; 2Rochester Unity, Rochester, NY; 3University of Missouri at Columbia, Columbia, MO; 4Hull University Teaching Hospitals, Hull, England, United Kingdom
Introduction: There remains inconsistent data about the efficacy of L-Ornithine L-Aspartate in managing hepatic encephalopathy as a stand-alone treatment in patients with cirrhosis of various etiologies. This systematic review and meta-analysis was conducted to determine LOLA’s effect compared to supportive management in improving mental status in patients with cirrhosis.
Methods: We searched Pubmed, EMBASE, and Google Scholar for randomized controlled trials evaluating L-Ornithine L-Aspartate as a stand-alone therapy and comparing it to placebo on patients with cirrhosis presenting with different grades of hepatic encephalopathy. We excluded studies that did not provide relevant data, studies that had compared the efficacy of LOLA as an add-on therapy, and unpublished trials. All analyses were done in R version 4.1.1 using the meta and metafor packages. The random effects model was used with an inverse variance approach for pooled prevalence and M-H method for calculating relative risk.
Results: Eventually, 11 RCTs were included in the quantitative analysis. Pooled data on the effects of LOLA as a stand-alone therapy on mental status improvement was assessed and showed significant benefit over placebo (Relative risk: 1.32, 1.12-1.55, I2 = 67%, p< 0.01). The improvement in mental status was evaluated by psychometric testing and/or West Haven Criteria, and reduction to at least one grade of hepatic encephalopathy from the presentation was included.
Pooled data from 9 RCTs in which venous ammonia levels were measured showed significant reductions following treatment with LOLA (Mean Difference -15.80, 95% CI -22.63 to -8.97, I2 =76%, p< 0.01) with intravenous or oral formulations compared to placebo/no intervention.
Discussion: There is significant efficacy in utilizing LOLA as a stand-alone therapy for improving mental status in patients with hepatic encephalopathy with cirrhosis. However, more research is needed in comparing the efficacy of LOLA against as well as along with the standard management. Also, only 4 trials mentioned the etiology of cirrhosis, and no subgroup data was available. More research needs to be done to see if there is a difference in the efficacy of LOLA with the etiology of cirrhosis and the different available formulations.

Figure: Forest Plot
Disclosures:
Mythili Menon Pathiyil indicated no relevant financial relationships.
Arvind Kumar indicated no relevant financial relationships.
Rutwik Pradeep Sharma indicated no relevant financial relationships.
Sanket Basida indicated no relevant financial relationships.
Jassimran Singh indicated no relevant financial relationships.
Sajjadh MJ Ali indicated no relevant financial relationships.
Shaji Sebastian indicated no relevant financial relationships.
Mythili Menon Pathiyil, MD1, Arvind Kumar, MD1, Rutwik Pradeep Sharma, MD2, Sanket Basida, MD3, Jassimran Singh, MD1, Sajjadh MJ Ali, MD1, Shaji Sebastian, MD4. P2400 - Efficacy of L-Ornithine L-Aspartate as a Stand-Alone Treatment for Hepatic Encephalopathy in Patients With Cirrhosis - A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
