Monday Poster Session
Category: Liver
P2407 - Semaglutide Outcomes in Non-Alcoholic Fatty Liver Disease: A Multi-Centered Real-World Study
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Wissam Ghusn, MD
Mayo Clinic
Boston, MA
Presenting Author(s)
Wissam Ghusn, MD1, Sima Fansa, MD1, Diego Anazco, MD1, Elif Tama, MD2, Bryan Nicolalde, MD1, Khushboo Gala, MBBS1, Lizeth Cifuentes, MD1, Alan De La Rosa, MD1, Daniel Sacoto, MD1, Alejandro Campos, MD1, Fauzi Feris, MD1, Andres Acosta, MD, PhD1
1Mayo Clinic, Rochester, MN; 2Mayo Clinic, Jacksonville, FL
Introduction: Obesity is a multifactorial disease with multiple associated comorbid condition including non-alcoholic fatty liver disease (NAFLD). Semaglutide is currently the most effective anti-obesity medication that demonstrated significant weight loss outcomes in patients with overweight or obesity. Limited data is present regarding the real-world effect of semaglutide on liver function tests (LFT). We aim to assess the 12-months weight loss and LFT outcomes of semaglutide in patients with NAFLD.
Methods: We performed a multi-centered cohort study of patients with NAFLD and BMI≥ 27 kg/m2 taking semaglutide for weight loss. Patients with history of bariatric surgery, taking other anti-obesity medications, and with active malignancy or pregnancy were excluded from this study. Our primary outcome included assessing the change in LFTs and secondary outcomes included evaluating total body weight loss percentage (TBWL%). We performed a paired t-test for our primary and secondary outcomes. Data are presented as mean ± standard deviation (SD).
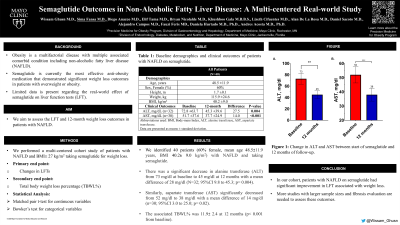
Results: WWe identified 40 patients (59% female, mean age 48.7± 11.8 years, BMI 40.1± 8.9 kg/m2) with NAFLD and taking semaglutide (Table 1). In our study, there was a significant decrease in alanine transferase (ALT) from 73 mg/dl at baseline to 45 mg/dl at 12 months with a mean difference of 28 mg/dl (n=32; 95%CI 9.8 to 45.3; p= 0.004; Figure 1A). Similarly, aspartate transferase (AST) significantly decreased from 52 mg/dl to 38 mg/dl with a mean difference of 14 mg/dl (n=30; 95%CI 3.0 to 25.0; p= 0.02; Figure 1B). The associated TBWL% was 11.9± 2.4 at 12 months (p< 0.001 from baseline).
Discussion: In our cohort, patients with NAFLD on semaglutide had significant improvement in LFT associated with weight loss. More studies with larger sample sizes and fibrosis evaluation are needed to assess these outcomes.

Disclosures:
Wissam Ghusn, MD1, Sima Fansa, MD1, Diego Anazco, MD1, Elif Tama, MD2, Bryan Nicolalde, MD1, Khushboo Gala, MBBS1, Lizeth Cifuentes, MD1, Alan De La Rosa, MD1, Daniel Sacoto, MD1, Alejandro Campos, MD1, Fauzi Feris, MD1, Andres Acosta, MD, PhD1. P2407 - Semaglutide Outcomes in Non-Alcoholic Fatty Liver Disease: A Multi-Centered Real-World Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Mayo Clinic, Jacksonville, FL
Introduction: Obesity is a multifactorial disease with multiple associated comorbid condition including non-alcoholic fatty liver disease (NAFLD). Semaglutide is currently the most effective anti-obesity medication that demonstrated significant weight loss outcomes in patients with overweight or obesity. Limited data is present regarding the real-world effect of semaglutide on liver function tests (LFT). We aim to assess the 12-months weight loss and LFT outcomes of semaglutide in patients with NAFLD.
Methods: We performed a multi-centered cohort study of patients with NAFLD and BMI≥ 27 kg/m2 taking semaglutide for weight loss. Patients with history of bariatric surgery, taking other anti-obesity medications, and with active malignancy or pregnancy were excluded from this study. Our primary outcome included assessing the change in LFTs and secondary outcomes included evaluating total body weight loss percentage (TBWL%). We performed a paired t-test for our primary and secondary outcomes. Data are presented as mean ± standard deviation (SD).
Results: WWe identified 40 patients (59% female, mean age 48.7± 11.8 years, BMI 40.1± 8.9 kg/m2) with NAFLD and taking semaglutide (Table 1). In our study, there was a significant decrease in alanine transferase (ALT) from 73 mg/dl at baseline to 45 mg/dl at 12 months with a mean difference of 28 mg/dl (n=32; 95%CI 9.8 to 45.3; p= 0.004; Figure 1A). Similarly, aspartate transferase (AST) significantly decreased from 52 mg/dl to 38 mg/dl with a mean difference of 14 mg/dl (n=30; 95%CI 3.0 to 25.0; p= 0.02; Figure 1B). The associated TBWL% was 11.9± 2.4 at 12 months (p< 0.001 from baseline).
Discussion: In our cohort, patients with NAFLD on semaglutide had significant improvement in LFT associated with weight loss. More studies with larger sample sizes and fibrosis evaluation are needed to assess these outcomes.

Figure: Figure 1: Change in ALT and AST between start of semaglutide and 12 months of follow-up.
Disclosures:
Wissam Ghusn indicated no relevant financial relationships.
Sima Fansa indicated no relevant financial relationships.
Diego Anazco indicated no relevant financial relationships.
Elif Tama indicated no relevant financial relationships.
Bryan Nicolalde indicated no relevant financial relationships.
Khushboo Gala indicated no relevant financial relationships.
Lizeth Cifuentes indicated no relevant financial relationships.
Alan De La Rosa indicated no relevant financial relationships.
Daniel Sacoto indicated no relevant financial relationships.
Alejandro Campos indicated no relevant financial relationships.
Fauzi Feris indicated no relevant financial relationships.
Andres Acosta: Amgen Pharmaceuticals – Consultant. General Mills – Consultant. Gila Therapeutics – Stock-privately held company. Phenomix Sciences – Stock-privately held company. Rhythm Pharmaceuticals – Consultant.
Wissam Ghusn, MD1, Sima Fansa, MD1, Diego Anazco, MD1, Elif Tama, MD2, Bryan Nicolalde, MD1, Khushboo Gala, MBBS1, Lizeth Cifuentes, MD1, Alan De La Rosa, MD1, Daniel Sacoto, MD1, Alejandro Campos, MD1, Fauzi Feris, MD1, Andres Acosta, MD, PhD1. P2407 - Semaglutide Outcomes in Non-Alcoholic Fatty Liver Disease: A Multi-Centered Real-World Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
