Monday Poster Session
Category: Liver
P2551 - A Rare Case of Idiosyncratic Metronidazole-Induced Severe Liver Injury
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Natasha Narang, DO
University of Arizona College of Medicine
Phoenix, AZ
Presenting Author(s)
Natasha Narang, DO, Victor Arce Gutierrez, MD, Paul Gomez, MD, Moises I. Nevah Rubin, MD
University of Arizona College of Medicine, Phoenix, AZ
Introduction: Metronidazole is a commonly prescribed antibiotic. Most frequently reported adverse effects include nausea, metallic taste and diarrhea. Clinically apparent liver injury due to metronidazole is extremely rare; less than 5 cases have been previously reported in the literature. We report a case of a patient who developed recurrent hepatocellular injury due to metronidazole.
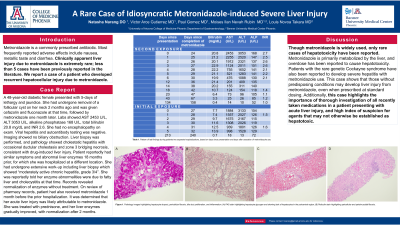
Case Description/Methods: A 48-year-old diabetic female presented with 5-days of lethargy and jaundice. She had undergone removal of a follicular cyst on her neck 2 months ago and was given cefazolin and fluconazole at that time, followed by metronidazole one month later. Labs showed AST 2453 U/L, ALT 3053 U/L, alkaline phosphatase 168 U/L, total bilirubin 20.8 mg/dL and INR 2.6. She had no encephalopathy on exam. Viral hepatitis and autoantibody testing was negative. Imaging showed no biliary obstruction. Liver biopsy was performed, and pathology showed cholestatic hepatitis with occasional ductular cholestasis and zone 3 bridging necrosis, consistent with drug-induced liver injury. Patient reportedly had similar symptoms and abnormal liver enzymes 18 months prior, for which she was hospitalized at a different location. She had undergone extensive work-up including liver biopsy which showed “moderately active chronic hepatitis, grade 3/4”. She was reportedly told her enzyme abnormalities were due to fatty liver and cholecystitis at that time. Records revealed normalization of enzymes without treatment. On review of pharmacy records, patient had also received metronidazole 1 month before the prior hospitalization. It was determined that her acute liver injury was likely attributable to metronidazole. She was treated with prednisone, and her liver enzymes gradually improved, with normalization after 2 months.
Discussion: Though metronidazole is widely used, only rare cases of hepatotoxicity have been reported. Metronidazole is primarily metabolized by the liver, and overdose has been reported to cause hepatotoxicity. Patients with the rare genetic Cockayne syndrome have also been reported to develop severe hepatitis with metronidazole use. This case shows that those without predisposing conditions may develop liver injury from metronidazole, even when prescribed at standard dosing. Additionally, this case highlights the importance of thorough investigation of all recently taken medications in a patient presenting with acute liver injury, and high index of suspicion for agents that may not otherwise be established as hepatotoxic.
Disclosures:
Natasha Narang, DO, Victor Arce Gutierrez, MD, Paul Gomez, MD, Moises I. Nevah Rubin, MD. P2551 - A Rare Case of Idiosyncratic Metronidazole-Induced Severe Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
University of Arizona College of Medicine, Phoenix, AZ
Introduction: Metronidazole is a commonly prescribed antibiotic. Most frequently reported adverse effects include nausea, metallic taste and diarrhea. Clinically apparent liver injury due to metronidazole is extremely rare; less than 5 cases have been previously reported in the literature. We report a case of a patient who developed recurrent hepatocellular injury due to metronidazole.
Case Description/Methods: A 48-year-old diabetic female presented with 5-days of lethargy and jaundice. She had undergone removal of a follicular cyst on her neck 2 months ago and was given cefazolin and fluconazole at that time, followed by metronidazole one month later. Labs showed AST 2453 U/L, ALT 3053 U/L, alkaline phosphatase 168 U/L, total bilirubin 20.8 mg/dL and INR 2.6. She had no encephalopathy on exam. Viral hepatitis and autoantibody testing was negative. Imaging showed no biliary obstruction. Liver biopsy was performed, and pathology showed cholestatic hepatitis with occasional ductular cholestasis and zone 3 bridging necrosis, consistent with drug-induced liver injury. Patient reportedly had similar symptoms and abnormal liver enzymes 18 months prior, for which she was hospitalized at a different location. She had undergone extensive work-up including liver biopsy which showed “moderately active chronic hepatitis, grade 3/4”. She was reportedly told her enzyme abnormalities were due to fatty liver and cholecystitis at that time. Records revealed normalization of enzymes without treatment. On review of pharmacy records, patient had also received metronidazole 1 month before the prior hospitalization. It was determined that her acute liver injury was likely attributable to metronidazole. She was treated with prednisone, and her liver enzymes gradually improved, with normalization after 2 months.
Discussion: Though metronidazole is widely used, only rare cases of hepatotoxicity have been reported. Metronidazole is primarily metabolized by the liver, and overdose has been reported to cause hepatotoxicity. Patients with the rare genetic Cockayne syndrome have also been reported to develop severe hepatitis with metronidazole use. This case shows that those without predisposing conditions may develop liver injury from metronidazole, even when prescribed at standard dosing. Additionally, this case highlights the importance of thorough investigation of all recently taken medications in a patient presenting with acute liver injury, and high index of suspicion for agents that may not otherwise be established as hepatotoxic.
Disclosures:
Natasha Narang indicated no relevant financial relationships.
Victor Arce Gutierrez indicated no relevant financial relationships.
Paul Gomez indicated no relevant financial relationships.
Moises I. Nevah Rubin indicated no relevant financial relationships.
Natasha Narang, DO, Victor Arce Gutierrez, MD, Paul Gomez, MD, Moises I. Nevah Rubin, MD. P2551 - A Rare Case of Idiosyncratic Metronidazole-Induced Severe Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
