Monday Poster Session
Category: Liver
P2585 - Suspected Drug-Induced Liver Injury with Autoimmune Features (AI-DILI) in a Patient Taking Liraglutide
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Ruchir Paladiya, MBBS
UConn Health
Hartford, CT
Presenting Author(s)
Ruchir Paladiya, MBBS1, Jasmine Tidwell, MD2, Niala Moallem, MD3, Neil D. Parikh, MD4
1UConn Health, Hartford, CT; 2UConn Health, Farmington, CT; 3University of Connecticut Health Center, Hartford, CT; 4Hartford Hospital, Hartford, CT
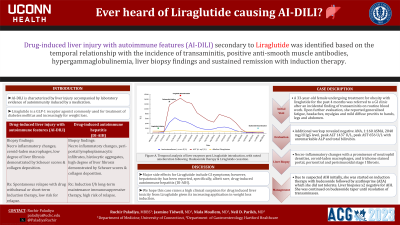
Introduction: Liraglutide is a GLP-1 receptor agonist commonly used for treatment of diabetes mellitus and increasingly for weight loss. We present a unique case of drug-induced liver injury with autoimmune features (AI-DILI) secondary to Liraglutide. AI-DILI is characterized by liver injury accompanied by laboratory evidence of autoimmunity induced by a medication. Key features include resolution of liver injury after the withdrawal of the offending medication and use of short-term immunosuppressive therapy, without relapse upon taper.
Case Description/Methods: A 33-year-old female undergoing treatment for obesity with liraglutide for the past 4 months was referred to a GI clinic after an incidental finding of transaminitis on routine blood work. Upon further evaluation, she reported generalized fatigue, headaches, myalgias and mild diffuse pruritis to hands, legs and abdomen. Additional workup revealed negative ANA, 1:160 ASMA, 2040 mg/dl IgG level, peak ALT 1437 U/L, peak AST 656 U/L with unremarkable ALP and total bilirubin. Due to suspected Autoimmune Hepatitis, she was started on budesonide. Liver biopsy showed necro-inflammatory changes with a prominence of neutrophil densities, ceroid-laden macrophages, and trichome stained portal, pericentral and perisinusoidal stage I fibrosis. After induction therapy with budesonide, she was started on azathioprine (AZA) due to suboptimal resolution of transaminitis. Secondary to GI side effects, AZA was discontinued after 20 days, and she was continued on a budesonide taper with eventual resolution of transaminitis over 3 months.
Discussion: There is limited literature on Liraglutide causing AI-DILI. Major side effects include GI symptoms; however, hepatotoxicity has been reported, specifically, albeit rare, drug-induced autoimmune hepatitis (DI-AIH). Our patient’s diagnosis of AI-DILI is based on the temporal relationship with the initiation of Liraglutide, transaminitis, positive ASMA, hypergammaglobulinemia, liver biopsy findings and sustained remission with induction therapy. Appropriate diagnosis of liver injury is critical as treatment varies. We hope this case raises a high clinical suspicion for drug induced liver toxicity from Liraglutide given its increasing application in weight loss induction.
Disclosures:
Ruchir Paladiya, MBBS1, Jasmine Tidwell, MD2, Niala Moallem, MD3, Neil D. Parikh, MD4. P2585 - Suspected Drug-Induced Liver Injury with Autoimmune Features (AI-DILI) in a Patient Taking Liraglutide, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1UConn Health, Hartford, CT; 2UConn Health, Farmington, CT; 3University of Connecticut Health Center, Hartford, CT; 4Hartford Hospital, Hartford, CT
Introduction: Liraglutide is a GLP-1 receptor agonist commonly used for treatment of diabetes mellitus and increasingly for weight loss. We present a unique case of drug-induced liver injury with autoimmune features (AI-DILI) secondary to Liraglutide. AI-DILI is characterized by liver injury accompanied by laboratory evidence of autoimmunity induced by a medication. Key features include resolution of liver injury after the withdrawal of the offending medication and use of short-term immunosuppressive therapy, without relapse upon taper.
Case Description/Methods: A 33-year-old female undergoing treatment for obesity with liraglutide for the past 4 months was referred to a GI clinic after an incidental finding of transaminitis on routine blood work. Upon further evaluation, she reported generalized fatigue, headaches, myalgias and mild diffuse pruritis to hands, legs and abdomen. Additional workup revealed negative ANA, 1:160 ASMA, 2040 mg/dl IgG level, peak ALT 1437 U/L, peak AST 656 U/L with unremarkable ALP and total bilirubin. Due to suspected Autoimmune Hepatitis, she was started on budesonide. Liver biopsy showed necro-inflammatory changes with a prominence of neutrophil densities, ceroid-laden macrophages, and trichome stained portal, pericentral and perisinusoidal stage I fibrosis. After induction therapy with budesonide, she was started on azathioprine (AZA) due to suboptimal resolution of transaminitis. Secondary to GI side effects, AZA was discontinued after 20 days, and she was continued on a budesonide taper with eventual resolution of transaminitis over 3 months.
Discussion: There is limited literature on Liraglutide causing AI-DILI. Major side effects include GI symptoms; however, hepatotoxicity has been reported, specifically, albeit rare, drug-induced autoimmune hepatitis (DI-AIH). Our patient’s diagnosis of AI-DILI is based on the temporal relationship with the initiation of Liraglutide, transaminitis, positive ASMA, hypergammaglobulinemia, liver biopsy findings and sustained remission with induction therapy. Appropriate diagnosis of liver injury is critical as treatment varies. We hope this case raises a high clinical suspicion for drug induced liver toxicity from Liraglutide given its increasing application in weight loss induction.
Disclosures:
Ruchir Paladiya indicated no relevant financial relationships.
Jasmine Tidwell indicated no relevant financial relationships.
Niala Moallem indicated no relevant financial relationships.
Neil Parikh indicated no relevant financial relationships.
Ruchir Paladiya, MBBS1, Jasmine Tidwell, MD2, Niala Moallem, MD3, Neil D. Parikh, MD4. P2585 - Suspected Drug-Induced Liver Injury with Autoimmune Features (AI-DILI) in a Patient Taking Liraglutide, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
