Monday Poster Session
Category: Stomach
P2722 - Gut Microbiome-Metabolome Signature in Helicobacter pylori Patients, Potential Implications in Progression of Disease and Treatment
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Hyder Alikhan, BA
Cooper Medical School of Rowan University
Camden, NJ
Presenting Author(s)
Hyder Alikhan, BA1, Brian White, MD2, John D.. Sterrett, BS3, Lamya'a M.. Dawud, PhD3, Marina N.. Farag, BA1, Christopher A.. Lowry, PhD3, Lark J.. Perez, PhD4, Joshua DeSipio, MD5, Sangita Phadtare, PhD1
1Cooper Medical School of Rowan University, Camden, NJ; 2University of Pennsylvania, Philadelphia, PA; 3University of Colorado Boulder, Boulder, CO; 4Rowan University, Glassboro, NJ; 5Cooper University Health Care, Camden, NJ
Introduction: Helicobacter pylori infects half of the world's population. There is increasing evidence that H. pylori can act outside the stomach by influencing the gut microbiota. We hypothesize that the interactions between H. pylori and the gut microbiome, and the resulting changes in the gut chemicals (bile acids and bacterial signaling molecules involved in quorum sensing, e.g., autoinducer-2/AI-2), influence pathogen survival, antibiotic response, and disease progression.
Methods: H. pylori patients and healthy controls were compared. Gut microbiome was analyzed with 16S rRNA gene sequencing, and Kruskal-Wallis test was used to assess differences in Faith's alpha diversity. Sparse correlation network investigation for compositional data analysis was used to cluster taxa at the species level into highly co-occurring modules. Differences in taxa were assessed by analysis of compositions of microbiomes with bias correction, controlling for age and sex. p-values from differential abundance testing were corrected for false discovery rate (FDR) using the Benjamini-Hochberg method. AI-2 concentrations were determined using AI-2 detecting, lux-producing bacteria. Bile acid concentrations were determined by liquid chromatography-mass spectrometry.
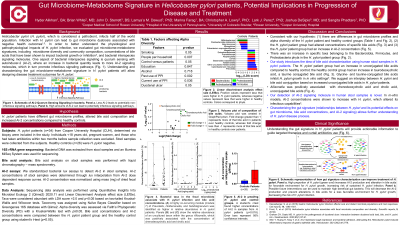
Results: H. pylori patients had: (i) decreased microbial alpha diversity (p=0.0018), (ii) increased AI-2 concentration (p=0.0186), and (iii) altered concentrations of certain bile acids (e.g., increased primary bile acids, chenodeoxycholic acid and cholic acid) compared to controls. Co-occurring module of Prevotella, Holdemanella, and Subdoligranulum, was higher in H. pylori patients (p=0.04). Allisonella was positively associated with chenodeoxycholic acid and cholic acid levels (p=0.05 and 0.02, respectively) (Figure 1).
Discussion: This is the first report characterizing the gut microbiome-metabolome signature (i.e., microbiome in context of bile acids and AI-2) in H. pylori patients. These three factors may account for significant H. pylori-mediated changes to the gut environment. Increased AI-2 may give selective advantage for growth of H. pylori further reducing microbial diversity. Cholic acid is shown to contribute to intestinal metaplasia and carcinogenesis. The interplay of these three factors may explain sustained H. pylori growth leading to antibiotic resistance and progression to cancer. Our study will provide actionable information that can guide targeted therapies and help curtail antibiotic usage.

Disclosures:
Hyder Alikhan, BA1, Brian White, MD2, John D.. Sterrett, BS3, Lamya'a M.. Dawud, PhD3, Marina N.. Farag, BA1, Christopher A.. Lowry, PhD3, Lark J.. Perez, PhD4, Joshua DeSipio, MD5, Sangita Phadtare, PhD1. P2722 - Gut Microbiome-Metabolome Signature in Helicobacter pylori Patients, Potential Implications in Progression of Disease and Treatment, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Cooper Medical School of Rowan University, Camden, NJ; 2University of Pennsylvania, Philadelphia, PA; 3University of Colorado Boulder, Boulder, CO; 4Rowan University, Glassboro, NJ; 5Cooper University Health Care, Camden, NJ
Introduction: Helicobacter pylori infects half of the world's population. There is increasing evidence that H. pylori can act outside the stomach by influencing the gut microbiota. We hypothesize that the interactions between H. pylori and the gut microbiome, and the resulting changes in the gut chemicals (bile acids and bacterial signaling molecules involved in quorum sensing, e.g., autoinducer-2/AI-2), influence pathogen survival, antibiotic response, and disease progression.
Methods: H. pylori patients and healthy controls were compared. Gut microbiome was analyzed with 16S rRNA gene sequencing, and Kruskal-Wallis test was used to assess differences in Faith's alpha diversity. Sparse correlation network investigation for compositional data analysis was used to cluster taxa at the species level into highly co-occurring modules. Differences in taxa were assessed by analysis of compositions of microbiomes with bias correction, controlling for age and sex. p-values from differential abundance testing were corrected for false discovery rate (FDR) using the Benjamini-Hochberg method. AI-2 concentrations were determined using AI-2 detecting, lux-producing bacteria. Bile acid concentrations were determined by liquid chromatography-mass spectrometry.
Results: H. pylori patients had: (i) decreased microbial alpha diversity (p=0.0018), (ii) increased AI-2 concentration (p=0.0186), and (iii) altered concentrations of certain bile acids (e.g., increased primary bile acids, chenodeoxycholic acid and cholic acid) compared to controls. Co-occurring module of Prevotella, Holdemanella, and Subdoligranulum, was higher in H. pylori patients (p=0.04). Allisonella was positively associated with chenodeoxycholic acid and cholic acid levels (p=0.05 and 0.02, respectively) (Figure 1).
Discussion: This is the first report characterizing the gut microbiome-metabolome signature (i.e., microbiome in context of bile acids and AI-2) in H. pylori patients. These three factors may account for significant H. pylori-mediated changes to the gut environment. Increased AI-2 may give selective advantage for growth of H. pylori further reducing microbial diversity. Cholic acid is shown to contribute to intestinal metaplasia and carcinogenesis. The interplay of these three factors may explain sustained H. pylori growth leading to antibiotic resistance and progression to cancer. Our study will provide actionable information that can guide targeted therapies and help curtail antibiotic usage.

Figure: Figure 1. Bacterial taxa in the fecal microbiota associate with H. pylori infection and bile acid concentrations. (A) A highly co-occurring module (module 7) of Prevotella, Holdemanella, and Subdoligranulum was identified as higher in relative abundance in H. pylori-infected individuals. Panels (B) and (C) show the relative abundance of an uncultured taxon within the genus Allisonella, which was positively associated with the concentration of chenodeoxycholic acid and cholic acid.
Disclosures:
Hyder Alikhan indicated no relevant financial relationships.
Brian White indicated no relevant financial relationships.
John Sterrett indicated no relevant financial relationships.
Lamya'a Dawud indicated no relevant financial relationships.
Marina Farag indicated no relevant financial relationships.
Christopher Lowry indicated no relevant financial relationships.
Lark Perez indicated no relevant financial relationships.
Joshua DeSipio indicated no relevant financial relationships.
Sangita Phadtare indicated no relevant financial relationships.
Hyder Alikhan, BA1, Brian White, MD2, John D.. Sterrett, BS3, Lamya'a M.. Dawud, PhD3, Marina N.. Farag, BA1, Christopher A.. Lowry, PhD3, Lark J.. Perez, PhD4, Joshua DeSipio, MD5, Sangita Phadtare, PhD1. P2722 - Gut Microbiome-Metabolome Signature in Helicobacter pylori Patients, Potential Implications in Progression of Disease and Treatment, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

