Monday Poster Session
Category: Stomach
P2732 - Glucagon-Like Peptide-1 Receptor Agonist Use Is Associated With an Increased Rate of Aborted Esophagogastroduodenoscopy and Need for Repeat EGD, Independent From the Presence of Diabetes
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Danial Nadeem, MD
Geisinger Medical Center
Bloomsburg, PA
Presenting Author(s)
Danial Nadeem, MD1, Mahdi Taye, BA2, James Dove, 1, Craig Wood, 1, David L. Diehl, MD1, Amitpal Johal, MD1, Christopher Still, DO1
1Geisinger Medical Center, Danville, PA; 2Geisinger Commonwealth School of Medicine, Scranton, PA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are prescribed for obesity and diabetes and help promote weight reduction by suppressing appetite, increasing satiety, and regulating glucose metabolism. However, an important adverse effect associated with GLP1-RAs is delayed gastric emptying, which can potentially impact endoscopic procedures such as esophagogastroduodenoscopy (EGD).
Methods: Endoscopy reports on patients aged 18 years or older who underwent outpatient EGD between January 1st, 2019 and June 1st, 2023 were reviewed. Data was extracted regarding 1. presence of food in the stomach at the time of EGD, 2. frequency of aborted procedures, 3. the need for repeat EGD within 30 days of the initial procedure due to an incomplete first EGD, and 4. any adverse event following EGD. In addition, demographic information was gathered including the prevalence of diabetes in these patients. The control group consisted of patients with no GLP-1 RA use within one year preceding EGD, while the exposure group included patients with an active GLP-1 RA prescription or documented GLP-1 RA use within 90 days prior to EGD.
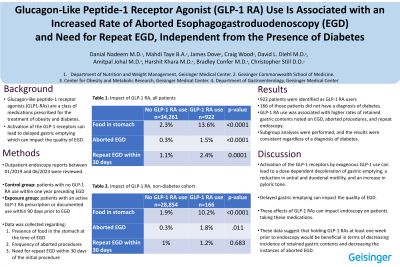
Results: In the study period, there were 35,183 EGDs performed, of which 922 patients were GLP-1 RA users. GLP-1 RA use was significantly associated with increased food in the stomach on EGD, increased rates of early EGD termination and need for repeat EGD within 30 days of initial procedure (p≤0.0001, Table 1a). There was no significant difference in post-procedural adverse events between the groups. Logistical regression demonstrated that GLP-1 RA users were four times more likely to have food in the stomach or have an aborted EGD (p < .0001), and twice as likely to require a repeat EGD within 30 days (p=.0021). After adjusting for concomitant diabetes in these patients, GLP-1 RA use was still significantly associated with increased food in the stomach, increased rates of early termination, as well as increased rates of repeat EGD (p≤ 0.0001, Table 1b).
Discussion: GLP-1 RAs are known to delay gastric emptying, and this may impact the quality of EGD. Even after controlling for diabetes, which can be associated with gastroparesis, GLP-1 RAs caused an increased incidence of retained gastric contents noted on EGD, resulting in more instances of aborted EGDs, and higher rates of repeat endoscopy due to incomplete examination. These effects of GLP-1 RAs should be considered when performing EGD on patients taking these medications.
Disclosures:
Danial Nadeem, MD1, Mahdi Taye, BA2, James Dove, 1, Craig Wood, 1, David L. Diehl, MD1, Amitpal Johal, MD1, Christopher Still, DO1. P2732 - Glucagon-Like Peptide-1 Receptor Agonist Use Is Associated With an Increased Rate of Aborted Esophagogastroduodenoscopy and Need for Repeat EGD, Independent From the Presence of Diabetes, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Geisinger Medical Center, Danville, PA; 2Geisinger Commonwealth School of Medicine, Scranton, PA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are prescribed for obesity and diabetes and help promote weight reduction by suppressing appetite, increasing satiety, and regulating glucose metabolism. However, an important adverse effect associated with GLP1-RAs is delayed gastric emptying, which can potentially impact endoscopic procedures such as esophagogastroduodenoscopy (EGD).
Methods: Endoscopy reports on patients aged 18 years or older who underwent outpatient EGD between January 1st, 2019 and June 1st, 2023 were reviewed. Data was extracted regarding 1. presence of food in the stomach at the time of EGD, 2. frequency of aborted procedures, 3. the need for repeat EGD within 30 days of the initial procedure due to an incomplete first EGD, and 4. any adverse event following EGD. In addition, demographic information was gathered including the prevalence of diabetes in these patients. The control group consisted of patients with no GLP-1 RA use within one year preceding EGD, while the exposure group included patients with an active GLP-1 RA prescription or documented GLP-1 RA use within 90 days prior to EGD.
Results: In the study period, there were 35,183 EGDs performed, of which 922 patients were GLP-1 RA users. GLP-1 RA use was significantly associated with increased food in the stomach on EGD, increased rates of early EGD termination and need for repeat EGD within 30 days of initial procedure (p≤0.0001, Table 1a). There was no significant difference in post-procedural adverse events between the groups. Logistical regression demonstrated that GLP-1 RA users were four times more likely to have food in the stomach or have an aborted EGD (p < .0001), and twice as likely to require a repeat EGD within 30 days (p=.0021). After adjusting for concomitant diabetes in these patients, GLP-1 RA use was still significantly associated with increased food in the stomach, increased rates of early termination, as well as increased rates of repeat EGD (p≤ 0.0001, Table 1b).
Discussion: GLP-1 RAs are known to delay gastric emptying, and this may impact the quality of EGD. Even after controlling for diabetes, which can be associated with gastroparesis, GLP-1 RAs caused an increased incidence of retained gastric contents noted on EGD, resulting in more instances of aborted EGDs, and higher rates of repeat endoscopy due to incomplete examination. These effects of GLP-1 RAs should be considered when performing EGD on patients taking these medications.
Disclosures:
Danial Nadeem indicated no relevant financial relationships.
Mahdi Taye indicated no relevant financial relationships.
James Dove indicated no relevant financial relationships.
Craig Wood indicated no relevant financial relationships.
David Diehl: Boston Scientific – Consultant. Castle – Consultant. Laborie – Consultant. Merit Endotek – Consultant. Microtech – Consultant. Olympus – Consultant. OnePass Medical – Consultant. Pentax – Consultant. Steris – Consultant.
Amitpal Johal indicated no relevant financial relationships.
Christopher Still: Novo Nordisk – Advisory Committee/Board Member, Speakers Bureau. Twenty30 Health – Advisor or Review Panel Member.
Danial Nadeem, MD1, Mahdi Taye, BA2, James Dove, 1, Craig Wood, 1, David L. Diehl, MD1, Amitpal Johal, MD1, Christopher Still, DO1. P2732 - Glucagon-Like Peptide-1 Receptor Agonist Use Is Associated With an Increased Rate of Aborted Esophagogastroduodenoscopy and Need for Repeat EGD, Independent From the Presence of Diabetes, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

