Monday Poster Session
Category: Stomach
P2741 - Efficacy of Endoscopic Intrapyloric Injection of Botulinum Toxin in the Treatment of Gastroparesis: A Systematic Review and Meta-Analysis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Rahul Karna, MD
Allegheny Health Network
Pittsburgh, Pennsylvania
Presenting Author(s)
Rahul Karna, MD1, Anand Kumar, MD2, Tanisha Kalra, MD3, Tabeer Rana, DO1, Muhammad Ali Butt, MD4, Taimur S. Muzammil, MD1, Babu Mohan, MD, MS5, Manish Dhawan, MD1, Douglas G. Adler, MD6
1Allegheny Health Network, Pittsburgh, PA; 2Lenox Hill Hospital, New York, NY; 3SUNY Downstate Health Sciences University/NYCHHC Kings County Hospital, New York, NY; 4Medicine Institute, Allegheny Health Network, Pittsburgh, PA; 5University of Utah Health School of Medicine, Salt Lake City, UT; 6Center for Advanced Therapeutic (CATE), Centura Health, Porter Adventist Hospital, Peak Gastroenterology, Denver, CO
Introduction: Observational studies suggest symptomatic improvement after intrapyloric injection of Botulinum toxin in treating gastroparesis in an attempt to paralyze the pyloric sphincter complex. However, a limited number of clinical trials failed to demonstrate any benefit to this therapy. We conducted a systematic review and meta-analysis to ascertain the outcomes of intrapyloric injection of Botulinum toxin in the treatment of gastroparesis.
Methods: We conducted a comprehensive search of Ovid Cochrane, Ovid Embase, Ovid Medline, Scopus, and Web of Science (inception to September 2022) to identify studies reporting on the use of intrapyloric injection of Botulinum toxin in gastroparesis. The primary outcome was overall clinical success while secondary outcomes were improvement in Gastroparesis Cardinal Symptom Index (GCSI), Gastric Emptying Study (GES); improvement in nausea/vomiting, fullness/distension, and bloating/belching. Clinical success was measured as improvement in either GES or GCSI. A meta-analysis of proportions was done for all primary and secondary outcomes.
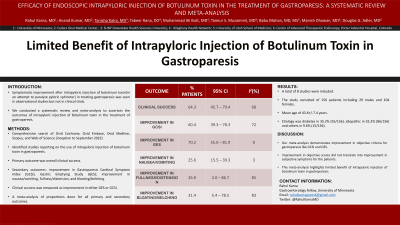
Results: A total of 8 studies were included. The study consisted of 156 patients including 29 males and 104 females; mean age of 43.6+/-7.4 years. Etiology was diabetes in 35.2% (55/156), idiopathic in 55.2% (86/156) and others in 9.6% (15/156). Overall clinical success was seen in 64.3% of patients (95% CI: 45.7-79.4%; I2 68%). Improvement in GCSI was seen in 60.4% of patients (95% CI: 39.3-78.3%; I2 72%) while improvement in GES was seen in 70.2% (95% CI: 55.0-81.9%; I2 0%). Symptomatic improvement in nausea/vomiting occurred in 25.6% (95% CI: 15.5-39.3%; I2 3%), fullness/distension occurred in 26.9% (95% CI: 2.0-86.7%; I2 85%) and bloating/belching occurred in 31.4% (95% CI: 5.4-78.5 %; I2 83%).
Discussion: Our meta-analysis demonstrates improvement in objective criteria for gastroparesis like GCSI and GES. However, improvement in objective scores did not translate into improvement in subjective symptoms for the patients. The study demonstrates a limited benefit of intrapyloric injection of Botulinum toxin in gastroparesis.
Disclosures:
Rahul Karna, MD1, Anand Kumar, MD2, Tanisha Kalra, MD3, Tabeer Rana, DO1, Muhammad Ali Butt, MD4, Taimur S. Muzammil, MD1, Babu Mohan, MD, MS5, Manish Dhawan, MD1, Douglas G. Adler, MD6. P2741 - Efficacy of Endoscopic Intrapyloric Injection of Botulinum Toxin in the Treatment of Gastroparesis: A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Allegheny Health Network, Pittsburgh, PA; 2Lenox Hill Hospital, New York, NY; 3SUNY Downstate Health Sciences University/NYCHHC Kings County Hospital, New York, NY; 4Medicine Institute, Allegheny Health Network, Pittsburgh, PA; 5University of Utah Health School of Medicine, Salt Lake City, UT; 6Center for Advanced Therapeutic (CATE), Centura Health, Porter Adventist Hospital, Peak Gastroenterology, Denver, CO
Introduction: Observational studies suggest symptomatic improvement after intrapyloric injection of Botulinum toxin in treating gastroparesis in an attempt to paralyze the pyloric sphincter complex. However, a limited number of clinical trials failed to demonstrate any benefit to this therapy. We conducted a systematic review and meta-analysis to ascertain the outcomes of intrapyloric injection of Botulinum toxin in the treatment of gastroparesis.
Methods: We conducted a comprehensive search of Ovid Cochrane, Ovid Embase, Ovid Medline, Scopus, and Web of Science (inception to September 2022) to identify studies reporting on the use of intrapyloric injection of Botulinum toxin in gastroparesis. The primary outcome was overall clinical success while secondary outcomes were improvement in Gastroparesis Cardinal Symptom Index (GCSI), Gastric Emptying Study (GES); improvement in nausea/vomiting, fullness/distension, and bloating/belching. Clinical success was measured as improvement in either GES or GCSI. A meta-analysis of proportions was done for all primary and secondary outcomes.
Results: A total of 8 studies were included. The study consisted of 156 patients including 29 males and 104 females; mean age of 43.6+/-7.4 years. Etiology was diabetes in 35.2% (55/156), idiopathic in 55.2% (86/156) and others in 9.6% (15/156). Overall clinical success was seen in 64.3% of patients (95% CI: 45.7-79.4%; I2 68%). Improvement in GCSI was seen in 60.4% of patients (95% CI: 39.3-78.3%; I2 72%) while improvement in GES was seen in 70.2% (95% CI: 55.0-81.9%; I2 0%). Symptomatic improvement in nausea/vomiting occurred in 25.6% (95% CI: 15.5-39.3%; I2 3%), fullness/distension occurred in 26.9% (95% CI: 2.0-86.7%; I2 85%) and bloating/belching occurred in 31.4% (95% CI: 5.4-78.5 %; I2 83%).
Discussion: Our meta-analysis demonstrates improvement in objective criteria for gastroparesis like GCSI and GES. However, improvement in objective scores did not translate into improvement in subjective symptoms for the patients. The study demonstrates a limited benefit of intrapyloric injection of Botulinum toxin in gastroparesis.
Disclosures:
Rahul Karna indicated no relevant financial relationships.
Anand Kumar indicated no relevant financial relationships.
Tanisha Kalra indicated no relevant financial relationships.
Tabeer Rana indicated no relevant financial relationships.
Muhammad Ali Butt indicated no relevant financial relationships.
Taimur Muzammil indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
Manish Dhawan indicated no relevant financial relationships.
Douglas Adler indicated no relevant financial relationships.
Rahul Karna, MD1, Anand Kumar, MD2, Tanisha Kalra, MD3, Tabeer Rana, DO1, Muhammad Ali Butt, MD4, Taimur S. Muzammil, MD1, Babu Mohan, MD, MS5, Manish Dhawan, MD1, Douglas G. Adler, MD6. P2741 - Efficacy of Endoscopic Intrapyloric Injection of Botulinum Toxin in the Treatment of Gastroparesis: A Systematic Review and Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
