Monday Poster Session
Category: Liver
P2530 - Semaglutide-Induced Hepatotoxicity: A Rare Case of Drug Induced Liver Injury
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- SG
Santiago F. Galeano Lovera, MD
Mayo Clinic Florida
Jacksonville, FL
Presenting Author(s)
Santiago F. Galeano Lovera, MD, Karthik Gnanapandithan, MD, MS
Mayo Clinic Florida, Jacksonville, FL
Introduction: Semaglutide is a GLP-1 analogue approved for the treatment of type 2 diabetes and weight loss. Drug-induced liver injury (DILI) is a rare but significant cause of liver disease associated with various medications. We present a case of a 67-year-old female who developed acute hepatocellular injury after initiation of semaglutide therapy for weight loss.
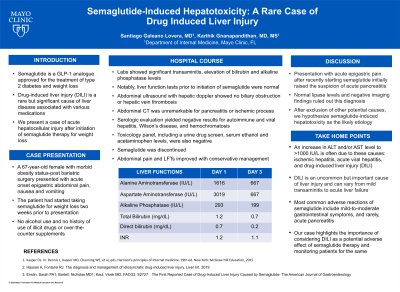
Case Description/Methods: A 67-year-old female with morbid obesity status-post bariatric surgery presented with acute onset epigastric abdominal pain, nausea and vomiting. She had started taking semaglutide two weeks back for weight loss. Laboratory tests revealed significant acute transaminitis (Alanine Transaminase 1616 IU/L, Aspartate Transaminase 3019 IU/L) with mild elevation of alkaline phosphatase (293 IU/L). Bilirubin, serum albumin, prothrombin time and lipase levels were normal. Liver function tests prior to initiation of semaglutide were normal. Abdominal ultrasound with hepatic doppler showed no signs of biliary obstruction or hepatic vein thrombosis. Abdominal computed tomography scan findings were unremarkable for pancreatitis or ischemic process. Serologic evaluation yielded negative results for autoimmune and viral hepatitis, Wilson’s disease, and hemochromatosis. Toxicology screen was negative. Due to concern for DILI, semaglutide was discontinued. Her transaminitis and abdominal pain showed significant improvement with conservative management. We hypothesize semaglutide-induced hepatotoxicity as the likely etiology based on clinical evidence and exclusion of other causes.
Discussion: DILI is an uncommon but important cause of liver injury. Semaglutide was approved in 2021 for weight management. It is also being considered in the management of non-alcoholic fatty liver disease, as it has been reported to reduce hepatic steatosis. Most common adverse reactions include mild-to-moderate gastrointestinal symptoms, and rarely, acute pancreatitis. To date, there is limited evidence of semaglutide-associated liver injury, with only one reported case in the literature. There have also been rare cases of liver injury associated with other GLP-1 analogues, such as liraglutide. Our case highlights the importance of considering DILI as a potential adverse effect of semaglutide therapy and monitoring patients for the same. With the increasing popularity of semaglutide for several metabolic disorders, clinicians need to be aware of this potential toxicity. Further investigation and larger scale studies are warranted to ensure its safety.
Disclosures:
Santiago F. Galeano Lovera, MD, Karthik Gnanapandithan, MD, MS. P2530 - Semaglutide-Induced Hepatotoxicity: A Rare Case of Drug Induced Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Mayo Clinic Florida, Jacksonville, FL
Introduction: Semaglutide is a GLP-1 analogue approved for the treatment of type 2 diabetes and weight loss. Drug-induced liver injury (DILI) is a rare but significant cause of liver disease associated with various medications. We present a case of a 67-year-old female who developed acute hepatocellular injury after initiation of semaglutide therapy for weight loss.
Case Description/Methods: A 67-year-old female with morbid obesity status-post bariatric surgery presented with acute onset epigastric abdominal pain, nausea and vomiting. She had started taking semaglutide two weeks back for weight loss. Laboratory tests revealed significant acute transaminitis (Alanine Transaminase 1616 IU/L, Aspartate Transaminase 3019 IU/L) with mild elevation of alkaline phosphatase (293 IU/L). Bilirubin, serum albumin, prothrombin time and lipase levels were normal. Liver function tests prior to initiation of semaglutide were normal. Abdominal ultrasound with hepatic doppler showed no signs of biliary obstruction or hepatic vein thrombosis. Abdominal computed tomography scan findings were unremarkable for pancreatitis or ischemic process. Serologic evaluation yielded negative results for autoimmune and viral hepatitis, Wilson’s disease, and hemochromatosis. Toxicology screen was negative. Due to concern for DILI, semaglutide was discontinued. Her transaminitis and abdominal pain showed significant improvement with conservative management. We hypothesize semaglutide-induced hepatotoxicity as the likely etiology based on clinical evidence and exclusion of other causes.
Discussion: DILI is an uncommon but important cause of liver injury. Semaglutide was approved in 2021 for weight management. It is also being considered in the management of non-alcoholic fatty liver disease, as it has been reported to reduce hepatic steatosis. Most common adverse reactions include mild-to-moderate gastrointestinal symptoms, and rarely, acute pancreatitis. To date, there is limited evidence of semaglutide-associated liver injury, with only one reported case in the literature. There have also been rare cases of liver injury associated with other GLP-1 analogues, such as liraglutide. Our case highlights the importance of considering DILI as a potential adverse effect of semaglutide therapy and monitoring patients for the same. With the increasing popularity of semaglutide for several metabolic disorders, clinicians need to be aware of this potential toxicity. Further investigation and larger scale studies are warranted to ensure its safety.
Disclosures:
Santiago Galeano Lovera indicated no relevant financial relationships.
Karthik Gnanapandithan indicated no relevant financial relationships.
Santiago F. Galeano Lovera, MD, Karthik Gnanapandithan, MD, MS. P2530 - Semaglutide-Induced Hepatotoxicity: A Rare Case of Drug Induced Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
