Monday Poster Session
Category: IBD
P2110 - Risk of Major Adverse Cardiovascular Events in Patients With Inflammatory Bowel Diseases or Other Immune-Mediated Inflammatory Disorders on Biologics and Small Molecules: A Systematic Review and Network Meta-Analysis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Shivani Mattay, MD
Washington University in St. Louis
St. Louis, MO
Presenting Author(s)
Award: Presidential Poster Award
Shivani Mattay, MD1, Mohammad Zamani, MD2, Dany Saturno, MD3, Edward V.. Loftus, MD4, Matthew Ciorba, MD1, Andres Yarur, MD5, Siddharth Singh, MD6, Parakkal Deepak, MBBS, MS7
1Washington University in St. Louis, St. Louis, MO; 2Tehran University of Medical Sciences, Tehran, Tehran, Iran; 3Washighton University, St. Louis, MO; 4Mayo Clinic College of Medicine and Science, Rochester, MN; 5Cedars-Sinai Medical Center, Los Angeles, CA; 6University of California, San Diego, San Diego, CA; 7Washington University in St. Louis School of Medicine, St. Louis, MO
Introduction: Recent studies raise concern for an increased risk of major adverse cardiovascular events (MACE) with Janus Kinase inhibitors (JAKi) used to treat immune-mediated inflammatory disorders (IMIDs). Our aim was to examine MACE risk with all licensed biologics and small molecules for patients with inflammatory bowel disease and other IMIDs such as psoriasis/Psoriatic arthritis and rheumatoid arthritis.
Methods: Data were obtained from systematic searches (from 1989 to May 31, 2022) in PubMed, Embase, Ovid Medline, Scopus, Cochrane Central, and Clinicaltrials.gov. Comparative studies that assessed a pre-defined MACE risk in patients with IMIDs during treatment with anti-interleukin-23 antibodies (anti-IL-23), anti-interleukin 12/23 antibodies (anti-IL-12/23), anti-tumor necrosis factor-alpha antibodies (anti-TNF-α) or JAKi were included. We performed a network meta-analysis using a random-effects model with results as pooled odds ratios (ORs) with 95% credible intervals (CrIs) according to drug class and disease state.
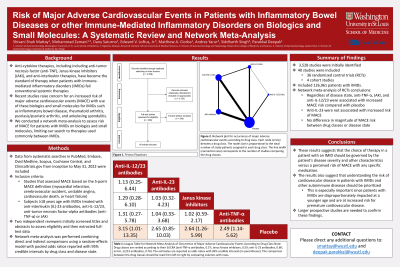
Results: Among 3,528 studies identified, 40 (including 36 randomized controlled trials [RCTs] and 4 cohort studies) were included in the systematic review, comprising 126,961 patients with IMIDs (Figure 1). Based on the network meta-analysis of RCTs, regardless of disease state, anti-TNF-α (OR, 2.49; 95% CrI: 1.14-5.62), JAKi (OR, 2.64; 95% CrI: 1.26-5.99), and anti-IL-12/23 (OR, 3.15; 95% CrI: 1.01-13.35) were associated with increased risk of MACE when compared to placebo, while anti-IL-23 (OR, 2.65; 95% CrI: 0.85-10.03) was not associated with an increased risk of MACE (Table 1). The magnitude of the increased risk of MACE with different drug classes was not significantly different when compared by IMID type.
Discussion: In this meta-analysis, we found that JAKi, anti-TNF-α, and anti-IL-12/23 increased the risk of MACE, while anti-IL-23 did not. We did not find a differential risk of MACE when stratified by disease state. Larger prospective studies are needed to confirm these findings.

Disclosures:
Shivani Mattay, MD1, Mohammad Zamani, MD2, Dany Saturno, MD3, Edward V.. Loftus, MD4, Matthew Ciorba, MD1, Andres Yarur, MD5, Siddharth Singh, MD6, Parakkal Deepak, MBBS, MS7. P2110 - Risk of Major Adverse Cardiovascular Events in Patients With Inflammatory Bowel Diseases or Other Immune-Mediated Inflammatory Disorders on Biologics and Small Molecules: A Systematic Review and Network Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Shivani Mattay, MD1, Mohammad Zamani, MD2, Dany Saturno, MD3, Edward V.. Loftus, MD4, Matthew Ciorba, MD1, Andres Yarur, MD5, Siddharth Singh, MD6, Parakkal Deepak, MBBS, MS7
1Washington University in St. Louis, St. Louis, MO; 2Tehran University of Medical Sciences, Tehran, Tehran, Iran; 3Washighton University, St. Louis, MO; 4Mayo Clinic College of Medicine and Science, Rochester, MN; 5Cedars-Sinai Medical Center, Los Angeles, CA; 6University of California, San Diego, San Diego, CA; 7Washington University in St. Louis School of Medicine, St. Louis, MO
Introduction: Recent studies raise concern for an increased risk of major adverse cardiovascular events (MACE) with Janus Kinase inhibitors (JAKi) used to treat immune-mediated inflammatory disorders (IMIDs). Our aim was to examine MACE risk with all licensed biologics and small molecules for patients with inflammatory bowel disease and other IMIDs such as psoriasis/Psoriatic arthritis and rheumatoid arthritis.
Methods: Data were obtained from systematic searches (from 1989 to May 31, 2022) in PubMed, Embase, Ovid Medline, Scopus, Cochrane Central, and Clinicaltrials.gov. Comparative studies that assessed a pre-defined MACE risk in patients with IMIDs during treatment with anti-interleukin-23 antibodies (anti-IL-23), anti-interleukin 12/23 antibodies (anti-IL-12/23), anti-tumor necrosis factor-alpha antibodies (anti-TNF-α) or JAKi were included. We performed a network meta-analysis using a random-effects model with results as pooled odds ratios (ORs) with 95% credible intervals (CrIs) according to drug class and disease state.
Results: Among 3,528 studies identified, 40 (including 36 randomized controlled trials [RCTs] and 4 cohort studies) were included in the systematic review, comprising 126,961 patients with IMIDs (Figure 1). Based on the network meta-analysis of RCTs, regardless of disease state, anti-TNF-α (OR, 2.49; 95% CrI: 1.14-5.62), JAKi (OR, 2.64; 95% CrI: 1.26-5.99), and anti-IL-12/23 (OR, 3.15; 95% CrI: 1.01-13.35) were associated with increased risk of MACE when compared to placebo, while anti-IL-23 (OR, 2.65; 95% CrI: 0.85-10.03) was not associated with an increased risk of MACE (Table 1). The magnitude of the increased risk of MACE with different drug classes was not significantly different when compared by IMID type.
Discussion: In this meta-analysis, we found that JAKi, anti-TNF-α, and anti-IL-12/23 increased the risk of MACE, while anti-IL-23 did not. We did not find a differential risk of MACE when stratified by disease state. Larger prospective studies are needed to confirm these findings.

Figure: Figure 1: Network plot for occurrence of major adverse cardiovascular events according to drug class. Each node (circle) denotes a drug class. The node size is proportional to the total number of study patients assigned to each drug class. The line width (connection size) corresponds to the number of studies comparing the drug classes.
Abbreviations: Abs, antibodies; IL, interleukin; JAK, Janus Kinase; TNF-α, tumor necrosis factor-alpha
Abbreviations: Abs, antibodies; IL, interleukin; JAK, Janus Kinase; TNF-α, tumor necrosis factor-alpha
Disclosures:
Shivani Mattay indicated no relevant financial relationships.
Mohammad Zamani indicated no relevant financial relationships.
Dany Saturno indicated no relevant financial relationships.
Edward Loftus: AbbVie – Consultant, Grant/Research Support. Alvotech – Consultant. Amgen – Consultant. Arena – Consultant. Avalo Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support. Celgene – Consultant, Grant/Research Support. Celltrion – Consultant. Exact Sciences – Stock-publicly held company(excluding mutual/index funds). Fresenius Kabi – Consultant. Genentech – Consultant, Grant/Research Support. Gilead – Consultant, Grant/Research Support. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant, Grant/Research Support. Iota Biosciences – Consultant. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support. KSL Diagnostics – Consultant. Lilly – Consultant. Morphic – Consultant. Ono Pharma – Consultant. Pfizer – Consultant, Grant/Research Support. Protagonist – Consultant. Receptos – Grant/Research Support. Robarts Clinical Trials – Grant/Research Support. Scipher Medicine – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Grant/Research Support. UCB – Consultant, Grant/Research Support.
Matthew Ciorba: AbbVie – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member.
Andres Yarur: BMS – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Pfizer – Consultant. Takeda – Consultant.
Siddharth Singh: Pfizer – Advisor or Review Panel Member, Grant/Research Support.
Parakkal Deepak: Abbvie – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Boehringer Ingelheim – Grant/Research Support. Fresenius Kabi – Consultant. Janssen – Grant/Research Support. Pfizer/Arena Pharmaceuticals – Grant/Research Support. Prometheus Biosciences – Grant/Research Support. Prometheus Labs – Grant/Research Support. Roche – Grant/Research Support, PI on a study. Sandoz – Advisory Committee/Board Member. Takeda – Grant/Research Support.
Shivani Mattay, MD1, Mohammad Zamani, MD2, Dany Saturno, MD3, Edward V.. Loftus, MD4, Matthew Ciorba, MD1, Andres Yarur, MD5, Siddharth Singh, MD6, Parakkal Deepak, MBBS, MS7. P2110 - Risk of Major Adverse Cardiovascular Events in Patients With Inflammatory Bowel Diseases or Other Immune-Mediated Inflammatory Disorders on Biologics and Small Molecules: A Systematic Review and Network Meta-Analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

