Tuesday Poster Session
Category: IBD
P3651 - Anti-CD20 Monoclonal Antibody Class Effect on Crohn’s Disease: A Case Series
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Alessandro Colletta, MD
UMass Chan Medical School
Worcester, MA
Presenting Author(s)
Alessandro Colletta, MD, Abbas Rupawala, MD
UMass Chan Medical School, Worcester, MA
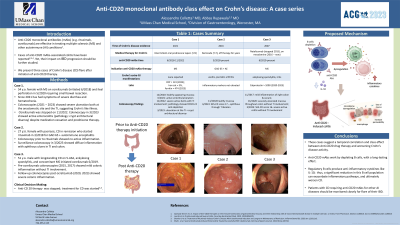
Introduction: Anti-CD20 monoclonal antibodies (mAbs) (e.g. rituximab, ocrelizumab) are effective in treating multiple sclerosis (MS) and other autoimmune (AI) conditions. Cases of anti-CD20 mAbs-associated colitis have been described; yet, there is limited data on their impact on the progression of inflammatory bowel disease (IBD). We herein present three cases of Crohn’s disease (CD) flare after initiation of anti-CD20 therapy.
Case Description/Methods: Case 1. 54-year-old female with MS on ocrelizumab (initiated 6/2018) and ileal perforation in 5/2020 requiring small bowel resection with ileocolonic anastomosis who since 2021 has had symptoms of severe diarrhea and hematochezia. Colonoscopies (2021 – 2023) showed severe ulceration both at the anastomotic site and the terminal ileum (TI), suggestive of Crohn’s like illness. Due to concern for Ocrelizumab-induced enterocolitis, the drug was stopped on 11/2022. Follow-up exam in 3/2023 showed active enterocolitis with pathology positive for crypt architectural disarray despite medication cessation and initiation of prednisone therapy.
Case 2. 27-year-old female with psoriasis, CD in remission who started rituximab in 3/2018 for GAD 65 + autoimmune encephalitis (AE). Colonoscopy prior to rituximab demonstrated no active inflammation. Surveillance colonoscopy in 3/2023 showed diffuse inflammation with aphthous ulcers in TI and colon with rectal sparing.
Case 3. 51-year-old-male with longstanding CD on 5-ASA, ankylosing spondylitis, and concomitant MS initiated ocrelizumab 6/2019. Pre-ocrelizumab colonoscopies (2015, 2017) showed mild colonic inflammation without TI involvement. Follow-up colonoscopies post ocrelizumab (2020, 2023) showed severe colonic inflammation. Stool calprotectin has also been elevated since starting ocrelizumab.
All three patients underwent discontinuation of the anti-CD 20 mAbs and are in the process of initiating treatment for their luminal Crohn’s disease.
Discussion: These cases suggest a temporal correlation and class effect between Anti-CD20 drug therapy and worsening Crohn’s disease activity. Anti-CD20 mAbs work by depleting B cells, and their effect can last for months. As regulatory B cells produce anti-inflammatory cytokines like IL-10, a significant reduction in this B cell population can exacerbate inflammatory pathways, disrupting gut homeostasis and worsening CD. Patients with Crohns disease requiring anti-CD20 mAbs for other AI disease should be monitored closely for flare of their IBD.
Disclosures:
Alessandro Colletta, MD, Abbas Rupawala, MD. P3651 - Anti-CD20 Monoclonal Antibody Class Effect on Crohn’s Disease: A Case Series, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
UMass Chan Medical School, Worcester, MA
Introduction: Anti-CD20 monoclonal antibodies (mAbs) (e.g. rituximab, ocrelizumab) are effective in treating multiple sclerosis (MS) and other autoimmune (AI) conditions. Cases of anti-CD20 mAbs-associated colitis have been described; yet, there is limited data on their impact on the progression of inflammatory bowel disease (IBD). We herein present three cases of Crohn’s disease (CD) flare after initiation of anti-CD20 therapy.
Case Description/Methods: Case 1. 54-year-old female with MS on ocrelizumab (initiated 6/2018) and ileal perforation in 5/2020 requiring small bowel resection with ileocolonic anastomosis who since 2021 has had symptoms of severe diarrhea and hematochezia. Colonoscopies (2021 – 2023) showed severe ulceration both at the anastomotic site and the terminal ileum (TI), suggestive of Crohn’s like illness. Due to concern for Ocrelizumab-induced enterocolitis, the drug was stopped on 11/2022. Follow-up exam in 3/2023 showed active enterocolitis with pathology positive for crypt architectural disarray despite medication cessation and initiation of prednisone therapy.
Case 2. 27-year-old female with psoriasis, CD in remission who started rituximab in 3/2018 for GAD 65 + autoimmune encephalitis (AE). Colonoscopy prior to rituximab demonstrated no active inflammation. Surveillance colonoscopy in 3/2023 showed diffuse inflammation with aphthous ulcers in TI and colon with rectal sparing.
Case 3. 51-year-old-male with longstanding CD on 5-ASA, ankylosing spondylitis, and concomitant MS initiated ocrelizumab 6/2019. Pre-ocrelizumab colonoscopies (2015, 2017) showed mild colonic inflammation without TI involvement. Follow-up colonoscopies post ocrelizumab (2020, 2023) showed severe colonic inflammation. Stool calprotectin has also been elevated since starting ocrelizumab.
All three patients underwent discontinuation of the anti-CD 20 mAbs and are in the process of initiating treatment for their luminal Crohn’s disease.
Discussion: These cases suggest a temporal correlation and class effect between Anti-CD20 drug therapy and worsening Crohn’s disease activity. Anti-CD20 mAbs work by depleting B cells, and their effect can last for months. As regulatory B cells produce anti-inflammatory cytokines like IL-10, a significant reduction in this B cell population can exacerbate inflammatory pathways, disrupting gut homeostasis and worsening CD. Patients with Crohns disease requiring anti-CD20 mAbs for other AI disease should be monitored closely for flare of their IBD.
Disclosures:
Alessandro Colletta indicated no relevant financial relationships.
Abbas Rupawala: Abbvie – Speakers Bureau. bristol Myers squibb – Advisory Committee/Board Member, Consultant.
Alessandro Colletta, MD, Abbas Rupawala, MD. P3651 - Anti-CD20 Monoclonal Antibody Class Effect on Crohn’s Disease: A Case Series, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
