Tuesday Poster Session
Category: IBD
P3674 - Rescue Therapy During Pregnancy With Ciclosporin in a Challenging Case of Ulcerative Colitis
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Narges Ashrafinia, MD
University of Saskatchewan
Saskatoon, SK, Canada
Presenting Author(s)
Narges Ashrafinia, MD1, Yvette Pui Yan Leung, MD, FRCPC2
1University of Saskatchewan, Saskatoon, SK, Canada; 2University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada
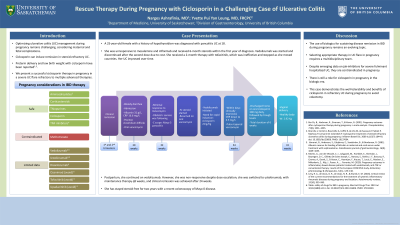
Introduction: Optimizing ulcerative colitis (UC) management during pregnancy remains challenging, considering maternal and fetal complications. Ciclosporin can induce remission in steroid-refractory UC. We present a successful ciclosporin therapy in pregnancy in a severe UC flare refractory to multiple advanced therapies.
Case Description/Methods: A 25-year-old female with a history of hypothyroidism was diagnosed with pancolitis UC at 18. She was unresponsive to mesalamine and infliximab and received 6-month steroids within the first year of diagnosis. Vedolizumab was started and discontinued after the second dose due to cost. She received a 2-month therapy with tofacitinib, which was ineffective and stopped as she moved countries. Her UC improved over time. Clinical remission was reported while she got pregnant and during her first and second trimesters of pregnancy without therapy. At the 30th gestational week, she was admitted with acute onset of frequent bloody, loose bowel movements in the context of clostridium difficile colitis. A 10-day oral vancomycin course was completed with minimal response. A colonoscopy showed Mayo 3 pancolitis. Intravenous steroids were started; oral vancomycin was continued. She was followed by obstetric service. Vedolizumab was initiated; however, given a need for a rapid induction agent, cyclosporin was started at 2mg/kg. Within a few days, her clinical symptoms improved, and the C-reactive protein level dropped from 16.6 mg/L to 3.5 mg/L (< 5.0 mg/L). Meanwhile, she received a pulse-tapered vancomycin regimen. She was discharged home on oral cyclosporin 300mg daily, followed by trough levels, for a total of 6 weeks. At 41 weeks of gestation, a healthy 2.92 kg baby was delivered via an uncomplicated vaginal delivery. Post-partum, she continued vedolizumab. Despite dose escalation, she was unresponsive. Ustekinumab was started with a transition to every 8-week subcutaneous dosing. After 24 weeks, she was in clinical remission. She has stayed steroid-free for two years with a recent colonoscopy of Mayo 0 disease.
Discussion: Selecting appropriate therapy in UC flare in pregnancy requires a multidisciplinary team. Despite emerging data on using jak-inhibitors for severe fulminant hospitalized UC, they are contraindicated in pregnancy. Hence, this case demonstrates there is still a role for ciclosporin in pregnancy.
Disclosures:
Narges Ashrafinia, MD1, Yvette Pui Yan Leung, MD, FRCPC2. P3674 - Rescue Therapy During Pregnancy With Ciclosporin in a Challenging Case of Ulcerative Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Saskatchewan, Saskatoon, SK, Canada; 2University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada
Introduction: Optimizing ulcerative colitis (UC) management during pregnancy remains challenging, considering maternal and fetal complications. Ciclosporin can induce remission in steroid-refractory UC. We present a successful ciclosporin therapy in pregnancy in a severe UC flare refractory to multiple advanced therapies.
Case Description/Methods: A 25-year-old female with a history of hypothyroidism was diagnosed with pancolitis UC at 18. She was unresponsive to mesalamine and infliximab and received 6-month steroids within the first year of diagnosis. Vedolizumab was started and discontinued after the second dose due to cost. She received a 2-month therapy with tofacitinib, which was ineffective and stopped as she moved countries. Her UC improved over time. Clinical remission was reported while she got pregnant and during her first and second trimesters of pregnancy without therapy. At the 30th gestational week, she was admitted with acute onset of frequent bloody, loose bowel movements in the context of clostridium difficile colitis. A 10-day oral vancomycin course was completed with minimal response. A colonoscopy showed Mayo 3 pancolitis. Intravenous steroids were started; oral vancomycin was continued. She was followed by obstetric service. Vedolizumab was initiated; however, given a need for a rapid induction agent, cyclosporin was started at 2mg/kg. Within a few days, her clinical symptoms improved, and the C-reactive protein level dropped from 16.6 mg/L to 3.5 mg/L (< 5.0 mg/L). Meanwhile, she received a pulse-tapered vancomycin regimen. She was discharged home on oral cyclosporin 300mg daily, followed by trough levels, for a total of 6 weeks. At 41 weeks of gestation, a healthy 2.92 kg baby was delivered via an uncomplicated vaginal delivery. Post-partum, she continued vedolizumab. Despite dose escalation, she was unresponsive. Ustekinumab was started with a transition to every 8-week subcutaneous dosing. After 24 weeks, she was in clinical remission. She has stayed steroid-free for two years with a recent colonoscopy of Mayo 0 disease.
Discussion: Selecting appropriate therapy in UC flare in pregnancy requires a multidisciplinary team. Despite emerging data on using jak-inhibitors for severe fulminant hospitalized UC, they are contraindicated in pregnancy. Hence, this case demonstrates there is still a role for ciclosporin in pregnancy.
Disclosures:
Narges Ashrafinia indicated no relevant financial relationships.
Yvette Pui Yan Leung: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Narges Ashrafinia, MD1, Yvette Pui Yan Leung, MD, FRCPC2. P3674 - Rescue Therapy During Pregnancy With Ciclosporin in a Challenging Case of Ulcerative Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
