Tuesday Poster Session
Category: Liver
P3809 - Utility of Novel FibroScan-Based Scores in the Diagnosis of Advanced Fibrosis in Nonalcoholic Fatty Liver Disease
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Ana Romero
Liver Associates of Texas, P.A., RWJ Barnabas Health/Trinitas Regional Medical Center
Houston, TX
Presenting Author(s)
Award: Presidential Poster Award
Ana Romero, 1, Jesus Romero, 1, Natalie Nguyen, 2, Oluseye Oniyide, 3, Javier Sanchez, 3, Chukwuma Egwim, MD4, Victor Ankoma-Sey, MD4
1Liver Associates of Texas, P.A., RWJ Barnabas Health/Trinitas Regional Medical Center, Houston, TX; 2Liver Associates of Texas, P.A., Baylor College of Medicine, Houston, TX; 3Liver Associates of Texas, P.A., Houston, TX; 4Liver Associates of Texas, P.A., Sherrie and Alan Conover Center for Liver Disease and Transplantation, JC Walter Jr Transplant Center, Houston Methodist Hospital, Houston, TX
Introduction: Non-alcoholic fatty liver disease (NAFLD) represents a public health concern. Multiple non-invasive biomarkers have been developed as possible screening tools for liver fibrosis in patients with NAFLD. Two new FibroScan-based scores, Agile 3+ and Agile 4 (combined liver stiffness measurement [LSM] by vibration-controlled transient elastography [VCTE] with blood biomarkers and clinical parameters) have been shown promising results. The aim of our study is to correlate these novel noninvasive score systems with FIB-4, a well-established scoring system, as predictors of hepatic fibrosis in patients with NAFLD.
Methods: A cross-sectional study was conducted at Liver Associates of Texas hepatology clinics in Houston, Texas. Included were patients diagnosed with NAFLD between September 2018 and May 2021. Liver stiffness measurements were obtained using transient elastography (FibroScan). Clinical parameters and blood biomarkers such as AST, ALT, platelets, age, sex, and diabetes status were collected. A binomial logistic regression model was used to determine the correlation of Agile 3+ and Agile 4 with FIB-4 score as predictors of advanced fibrosis. Advanced fibrosis (≥ F3) was defined as a FIB-4 score > 2.67. A p-value of less than 0.05 was considered statistically significant.
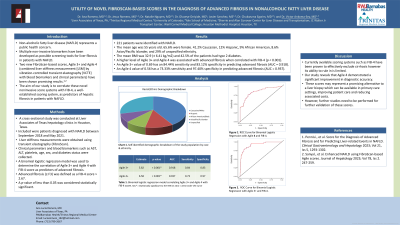
Results: 221 patients were identified with NAFLD. The mean age was 55 years old. 65.6% were female. 41.2% Caucasian, 12% Hispanic, 9% African American, 8.6% Asian/Pacific Islander, and 29% of unspecified ethnicity. The mean BMI was 32.9 (± 6.41 kg/m2) and 42.5% of the patients had type 2 diabetes. A higher level of Agile 3+ and Agile 4 was associated with advanced fibrosis when correlated with FIB-4 (p < 0.001). An Agile 3+ value of 0.69 has an 84.44% sensitivity and 83.12% specificity in predicting advanced fibrosis (AUC = 0.918). An Agile 4 value of 0.56 has a 73.33% sensitivity and 97.40% specificity in predicting advanced fibrosis (AUC = 0.937).
Discussion: Currently available scoring systems such as FIB-4 have been proven to effectively exclude cirrhosis however its ability to rule in is limited. Our study reveals that Agile 4 demonstrated a significant improvement in diagnostic accuracy. These scores may represent a promising alternative to a liver biopsy which can be available in primary care settings, improving patient care and reducing associated costs. However, further studies need to be performed for further validation of these scores.

Disclosures:
Ana Romero, 1, Jesus Romero, 1, Natalie Nguyen, 2, Oluseye Oniyide, 3, Javier Sanchez, 3, Chukwuma Egwim, MD4, Victor Ankoma-Sey, MD4. P3809 - Utility of Novel FibroScan-Based Scores in the Diagnosis of Advanced Fibrosis in Nonalcoholic Fatty Liver Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Ana Romero, 1, Jesus Romero, 1, Natalie Nguyen, 2, Oluseye Oniyide, 3, Javier Sanchez, 3, Chukwuma Egwim, MD4, Victor Ankoma-Sey, MD4
1Liver Associates of Texas, P.A., RWJ Barnabas Health/Trinitas Regional Medical Center, Houston, TX; 2Liver Associates of Texas, P.A., Baylor College of Medicine, Houston, TX; 3Liver Associates of Texas, P.A., Houston, TX; 4Liver Associates of Texas, P.A., Sherrie and Alan Conover Center for Liver Disease and Transplantation, JC Walter Jr Transplant Center, Houston Methodist Hospital, Houston, TX
Introduction: Non-alcoholic fatty liver disease (NAFLD) represents a public health concern. Multiple non-invasive biomarkers have been developed as possible screening tools for liver fibrosis in patients with NAFLD. Two new FibroScan-based scores, Agile 3+ and Agile 4 (combined liver stiffness measurement [LSM] by vibration-controlled transient elastography [VCTE] with blood biomarkers and clinical parameters) have been shown promising results. The aim of our study is to correlate these novel noninvasive score systems with FIB-4, a well-established scoring system, as predictors of hepatic fibrosis in patients with NAFLD.
Methods: A cross-sectional study was conducted at Liver Associates of Texas hepatology clinics in Houston, Texas. Included were patients diagnosed with NAFLD between September 2018 and May 2021. Liver stiffness measurements were obtained using transient elastography (FibroScan). Clinical parameters and blood biomarkers such as AST, ALT, platelets, age, sex, and diabetes status were collected. A binomial logistic regression model was used to determine the correlation of Agile 3+ and Agile 4 with FIB-4 score as predictors of advanced fibrosis. Advanced fibrosis (≥ F3) was defined as a FIB-4 score > 2.67. A p-value of less than 0.05 was considered statistically significant.
Results: 221 patients were identified with NAFLD. The mean age was 55 years old. 65.6% were female. 41.2% Caucasian, 12% Hispanic, 9% African American, 8.6% Asian/Pacific Islander, and 29% of unspecified ethnicity. The mean BMI was 32.9 (± 6.41 kg/m2) and 42.5% of the patients had type 2 diabetes. A higher level of Agile 3+ and Agile 4 was associated with advanced fibrosis when correlated with FIB-4 (p < 0.001). An Agile 3+ value of 0.69 has an 84.44% sensitivity and 83.12% specificity in predicting advanced fibrosis (AUC = 0.918). An Agile 4 value of 0.56 has a 73.33% sensitivity and 97.40% specificity in predicting advanced fibrosis (AUC = 0.937).
Discussion: Currently available scoring systems such as FIB-4 have been proven to effectively exclude cirrhosis however its ability to rule in is limited. Our study reveals that Agile 4 demonstrated a significant improvement in diagnostic accuracy. These scores may represent a promising alternative to a liver biopsy which can be available in primary care settings, improving patient care and reducing associated costs. However, further studies need to be performed for further validation of these scores.

Figure: Figure 1. ROC curve for Agile 4 score.
Disclosures:
Ana Romero indicated no relevant financial relationships.
Jesus Romero indicated no relevant financial relationships.
Natalie Nguyen indicated no relevant financial relationships.
Oluseye Oniyide indicated no relevant financial relationships.
Javier Sanchez indicated no relevant financial relationships.
Chukwuma Egwim: AbbVie – Sub-Investigator. Akero Therapeutics, Inc. – Sub-Investigator. Altimmune, Inc. – Sub-Investigator. Escient Pharmaceuticals, Inc. – Sub-Investigator. Gilead Sciences, Inc. – Sub-Investigator. GlaxoSmithKline Research & Development Limited – Sub-Investigator. Intercept Pharmaceuticals, Inc. – Sub-Investigator. Inventiva S.A. – Sub-Investigator. Ipsen Bioscience, Inc. – Sub-Investigator. Madrigal Pharmaceuticals, Inc. – Sub-Investigator. Mirum Pharmaceuticals, Inc. – Sub-Investigator. Regeneron Pharmaceuticals, Inc. – Sub-Investigator. Salix Pharmaceuticals, Inc. – Sub-Investigator. Zydus Therapeutics, Inc. – Sub-Investigator.
Victor Ankoma-Sey: AbbVie – Principal Investigator. Akero Therapeutics, Inc. – Principal Investigator. Altimmune, Inc. – Principal Investigator. Escient Pharmaceuticals, Inc. – Principal Investigator. Gilead Sciences, Inc. – Principal Investigator. GlaxoSmithKline Research & Development Limited – Principal Investigator. Intercept Pharmaceuticals, Inc. – Principal Investigator. Inventiva S.A. – Principal Investigator. Ipsen Bioscience, Inc. – Principal Investigator. Madrigal Pharmaceuticals, Inc. – Principal Investigator. Mirum Pharmaceuticals, Inc. – Principal Investigator. Regeneron Pharmaceuticals, Inc. – Principal Investigator. Salix Pharmaceuticals, Inc. – Principal Investigator. Zydus Therapeutics, Inc. – Principal Investigator.
Ana Romero, 1, Jesus Romero, 1, Natalie Nguyen, 2, Oluseye Oniyide, 3, Javier Sanchez, 3, Chukwuma Egwim, MD4, Victor Ankoma-Sey, MD4. P3809 - Utility of Novel FibroScan-Based Scores in the Diagnosis of Advanced Fibrosis in Nonalcoholic Fatty Liver Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

