Tuesday Poster Session
Category: Liver
P3817 - Dynamic Limonene Breath Testing Maximizes Classification Performance for Subjects With Cirrhosis
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- GF
Giuseppe Ferrandino
Owlstone Medical
Cambridge, England, United Kingdom
Presenting Author(s)
Giuseppe Ferrandino, 1, Federico Ricciardi, 1, Antonio Murgia, 1, Iris Banda, 1, Yusuf Ahmed, 1, Menisha Manhota, 1, Kelly Sweeney, 1, Louise Nicholson-Scott, BSc1, Lucinda McConville, 1, Olga Gandelman, MBA1, Max Allsworth, 1, Billy Boyle, 1, Agnieszka Smolinska, 1, Alexandra Ginesta Frings, 2, Jorge Contreras Basulto, 3, Claudia Asenjo-Lobos, 4, Viviana Barrientos Parra, 5, Nataly Clavo Morales, 5, Angela Novoa Arce, 6, Amy Riviotta, 4, Melissa Javiera Jerez Pardo, 7, Luis Méndez Alcamán, 8
1Owlstone Medical, Cambridge, England, United Kingdom; 2Gastroenterología, Clínica Alemana, Facultad de Medicina Clínica Alemana Universidad de Desarrollo, Santiago, Chile.Unidad de endoscopia, Hospital Padre Hurtado, Santiago, Chile., Santiago, Region Metropolitana, Chile; 3Gastroenterología, Clínica Alemana, Facultad de Medicina Clínica Alemana Universidad de Desarrollo, Santiago, Region Metropolitana, Chile; 4Centro de Estudios Clínicos, Instituto de Ciencias e Innovación en Medicina (ICIM), Facultad de Medicina Clínica Alemana Universidad del Desarrollo, Santiago, Region Metropolitana, Chile; 5Unidad de Endoscopia, Hospital Padre Hurtado, Santiago, Region Metropolitana, Chile; 6Laboratorio de Fisiología Digestiva, Clínica Alemana, Santiago, Region Metropolitana, Chile; 7Universidad de Las Américas, Santiago, Region Metropolitana, Chile; 8Gastroenterología, Clínica Alemana, Facultad de Medicina Clínica Alemana Universidad de Desarrollo, Unidad de endoscopia, Hospital Padre Hurtado, Santiago, Region Metropolitana, Chile
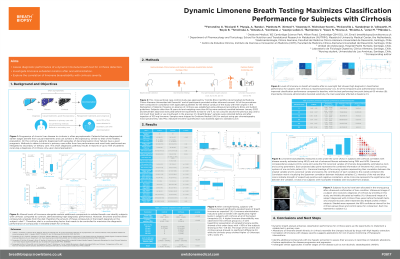
Introduction: The current diagnostic pathway for liver cirrhosis results in up to 75% of the cases diagnosed at advanced stages with decompensation. Chronic liver diseases alter the systemic bioavailability of xenobiotics undergoing phase 1 metabolism. Limonene, one of these xenobiotics, is partially excreted unchanged in the breath, where it can be non-invasively measured to assess alterations associated with chronic liver disease. With an unprecedented study design, dynamic limonene breath analysis was evaluated for classification performance for cirrhosis, and correlations with risk of liver fibrosis and disease severity.
Methods: A total of 29 subjects with cirrhosis [Child-Pugh: 22 A, 5 B, 2 NAs] and 29 controls [17 f, 12 m], were enrolled with random recruitment, and orally administered 100 mg limonene after an overnight fasting. Breath samples were collected before, and 20, 40, 60, 90, and 120 minutes after ingestion using the ReCIVA® breath sampler. Absolute limonene quantification was obtained by gas chromatography mass spectrometry. Blood tests were administered within 6 months from breath sampling. Logistic regression with cross-validation was used to assess classification performance. Correlation of breath limonene with risk of fibrosis and disease severity was evaluated using canonical correlation analysis (CCA).
Results: All tested timepoints, showed higher levels of breath limonene in subjects with cirrhosis compared to controls (p < 0.01). At baseline limonene discriminated subjects with cirrhosis from controls with an AUC (SD) of 0.87 (0.11). Limonene administration induced a spike on breath of >100 folds with a peak (Cmax) after 20 or 40 minutes (Tmax) in more than 90% of the subjects. AUCs (sd) at post administration timepoints were respectively: 0.89 (0.10); 0.93 (0.06) (Fig. 1A); 0.91 (0.08); 0.92 (0.07); 0.94 (0.06).
In subjects with cirrhosis, breath limonene levels at Cmax showed a collective correlation with liver fibrosis risk estimated with FIB4 and APRI, and disease severity estimated with MELD score [R2 = 0.76, p < 0.001] (Fig. 1B). No correlations were observed in the control group.
Discussion: The dynamic limonene breath analysis demonstrates excellent performance to discriminate cirrhosis. It may also serve to identify fibrosis and monitor disease regression after therapeutic intervention. This premise should be evaluated with/against Fibroscan in patients with various stages and etiologies.

Disclosures:
Giuseppe Ferrandino, 1, Federico Ricciardi, 1, Antonio Murgia, 1, Iris Banda, 1, Yusuf Ahmed, 1, Menisha Manhota, 1, Kelly Sweeney, 1, Louise Nicholson-Scott, BSc1, Lucinda McConville, 1, Olga Gandelman, MBA1, Max Allsworth, 1, Billy Boyle, 1, Agnieszka Smolinska, 1, Alexandra Ginesta Frings, 2, Jorge Contreras Basulto, 3, Claudia Asenjo-Lobos, 4, Viviana Barrientos Parra, 5, Nataly Clavo Morales, 5, Angela Novoa Arce, 6, Amy Riviotta, 4, Melissa Javiera Jerez Pardo, 7, Luis Méndez Alcamán, 8. P3817 - Dynamic Limonene Breath Testing Maximizes Classification Performance for Subjects With Cirrhosis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Owlstone Medical, Cambridge, England, United Kingdom; 2Gastroenterología, Clínica Alemana, Facultad de Medicina Clínica Alemana Universidad de Desarrollo, Santiago, Chile.Unidad de endoscopia, Hospital Padre Hurtado, Santiago, Chile., Santiago, Region Metropolitana, Chile; 3Gastroenterología, Clínica Alemana, Facultad de Medicina Clínica Alemana Universidad de Desarrollo, Santiago, Region Metropolitana, Chile; 4Centro de Estudios Clínicos, Instituto de Ciencias e Innovación en Medicina (ICIM), Facultad de Medicina Clínica Alemana Universidad del Desarrollo, Santiago, Region Metropolitana, Chile; 5Unidad de Endoscopia, Hospital Padre Hurtado, Santiago, Region Metropolitana, Chile; 6Laboratorio de Fisiología Digestiva, Clínica Alemana, Santiago, Region Metropolitana, Chile; 7Universidad de Las Américas, Santiago, Region Metropolitana, Chile; 8Gastroenterología, Clínica Alemana, Facultad de Medicina Clínica Alemana Universidad de Desarrollo, Unidad de endoscopia, Hospital Padre Hurtado, Santiago, Region Metropolitana, Chile
Introduction: The current diagnostic pathway for liver cirrhosis results in up to 75% of the cases diagnosed at advanced stages with decompensation. Chronic liver diseases alter the systemic bioavailability of xenobiotics undergoing phase 1 metabolism. Limonene, one of these xenobiotics, is partially excreted unchanged in the breath, where it can be non-invasively measured to assess alterations associated with chronic liver disease. With an unprecedented study design, dynamic limonene breath analysis was evaluated for classification performance for cirrhosis, and correlations with risk of liver fibrosis and disease severity.
Methods: A total of 29 subjects with cirrhosis [Child-Pugh: 22 A, 5 B, 2 NAs] and 29 controls [17 f, 12 m], were enrolled with random recruitment, and orally administered 100 mg limonene after an overnight fasting. Breath samples were collected before, and 20, 40, 60, 90, and 120 minutes after ingestion using the ReCIVA® breath sampler. Absolute limonene quantification was obtained by gas chromatography mass spectrometry. Blood tests were administered within 6 months from breath sampling. Logistic regression with cross-validation was used to assess classification performance. Correlation of breath limonene with risk of fibrosis and disease severity was evaluated using canonical correlation analysis (CCA).
Results: All tested timepoints, showed higher levels of breath limonene in subjects with cirrhosis compared to controls (p < 0.01). At baseline limonene discriminated subjects with cirrhosis from controls with an AUC (SD) of 0.87 (0.11). Limonene administration induced a spike on breath of >100 folds with a peak (Cmax) after 20 or 40 minutes (Tmax) in more than 90% of the subjects. AUCs (sd) at post administration timepoints were respectively: 0.89 (0.10); 0.93 (0.06) (Fig. 1A); 0.91 (0.08); 0.92 (0.07); 0.94 (0.06).
In subjects with cirrhosis, breath limonene levels at Cmax showed a collective correlation with liver fibrosis risk estimated with FIB4 and APRI, and disease severity estimated with MELD score [R2 = 0.76, p < 0.001] (Fig. 1B). No correlations were observed in the control group.
Discussion: The dynamic limonene breath analysis demonstrates excellent performance to discriminate cirrhosis. It may also serve to identify fibrosis and monitor disease regression after therapeutic intervention. This premise should be evaluated with/against Fibroscan in patients with various stages and etiologies.

Figure: Fig. 1
Disclosures:
Giuseppe Ferrandino: Owlstone Medical – Employee.
Federico Ricciardi: Owlstone Medical – Employee.
Antonio Murgia: Owlstone Medical – Employee. Owlstone Medical – Employee.
Iris Banda: Owlstone Medical – Employee.
Yusuf Ahmed: Owlstone Medical – Employee.
Menisha Manhota: Owlstone Medical – Employee.
Kelly Sweeney: Owlstone Medical – Employee.
Louise Nicholson-Scott: Owlstone Medical – Employee.
Lucinda McConville: Owlstone Medical – Employee.
Olga Gandelman: Owlstone Medical – Employee.
Max Allsworth: Owlstone Medical – Employee.
Billy Boyle: Owlstone Medical – Employee.
Agnieszka Smolinska: Owlstone Medical – Employee.
Alexandra Ginesta Frings indicated no relevant financial relationships.
Jorge Contreras Basulto indicated no relevant financial relationships.
Claudia Asenjo-Lobos indicated no relevant financial relationships.
Viviana Barrientos Parra indicated no relevant financial relationships.
Nataly Clavo Morales indicated no relevant financial relationships.
Angela Novoa Arce indicated no relevant financial relationships.
Amy Riviotta indicated no relevant financial relationships.
Melissa Javiera Jerez Pardo indicated no relevant financial relationships.
Luis Méndez Alcamán indicated no relevant financial relationships.
Giuseppe Ferrandino, 1, Federico Ricciardi, 1, Antonio Murgia, 1, Iris Banda, 1, Yusuf Ahmed, 1, Menisha Manhota, 1, Kelly Sweeney, 1, Louise Nicholson-Scott, BSc1, Lucinda McConville, 1, Olga Gandelman, MBA1, Max Allsworth, 1, Billy Boyle, 1, Agnieszka Smolinska, 1, Alexandra Ginesta Frings, 2, Jorge Contreras Basulto, 3, Claudia Asenjo-Lobos, 4, Viviana Barrientos Parra, 5, Nataly Clavo Morales, 5, Angela Novoa Arce, 6, Amy Riviotta, 4, Melissa Javiera Jerez Pardo, 7, Luis Méndez Alcamán, 8. P3817 - Dynamic Limonene Breath Testing Maximizes Classification Performance for Subjects With Cirrhosis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
