Tuesday Poster Session
Category: Liver
P3819 - Impact of Patient Database Review on Hepatocellular Carcinoma Surveillance Rates in a Veteran Population
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Jordan L. Redfield, MD
University of Tennessee Health Science Center
Memphis, TN
Presenting Author(s)
Jordan L.. Redfield, MD1, Samantha A.. Whitwell, MD1, Lorri Reaves, RN2, Claudio R.. Tombazzi, MD2
1University of Tennessee Health Science Center, Memphis, TN; 2Memphis Veterans Affairs Medical Center, Memphis, TN
Introduction: Per the American Association for the Study of Liver Diseases (AASLD), hepatocellular carcinoma (HCC) surveillance with ultrasonography (US) every six months is recommended for patients at high risk for HCC. High-risk patients include those with cirrhosis and many with chronic hepatitis B virus (HBV). HCC surveillance serves to detect HCC at an early stage, offering a more favorable prognosis. Unfortunately, many patients who qualify do not receive recommended HCC surveillance. This study sought to improve the number of patients at the Memphis Veterans Affairs Medical Center (VAMC) with cirrhosis or chronic HBV compliant with HCC surveillance.
Methods: A retrospective chart analysis of an existing patient database was performed. Three hundred patients were randomly selected from a database of 884 patients whose chart data suggested a known or possible diagnosis of cirrhosis or chronic HBV. For patients with no evidence of cirrhosis or chronic HBV upon review, charts were removed from the database. For patients with cirrhosis or chronic HBV, charts were evaluated for compliance with HCC surveillance imaging. If imaging had not been obtained within the past six months, US (or CT or MRI, as appropriate) was ordered. This chart review and intervention occurred over the course of one year; one month after completion, follow-up chart review was performed to determine if patients who were originally non-compliant with HCC surveillance were now compliant.
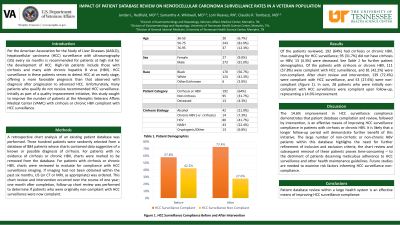
Results: 192 patients (64%) had cirrhosis or chronic HBV; 95 (31.7%) did not have cirrhosis or HBV; 13 (4.3%) were deceased. Of the patients with cirrhosis or chronic HBV, 111 (57.8%) were complaint with HCC surveillance, and 81 (42.2%) were non-compliant. After chart review and intervention, 139 (72.4%) were compliant with HCC surveillance, and 53 (27.6%) were non-compliant. In sum, 28 patients who were initially non-compliant with HCC surveillance were compliant upon follow-up, a 14.6% improvement.
Discussion: Database compilation and review within a large health system is an effective means of improving HCC surveillance rates in patients with cirrhosis or chronic HBV. It is likely that a longer follow-up period will demonstrate further benefit of this initiative. The large number of non-cirrhotic or non-chronic HBV patients within this database highlights the need for further refinement of inclusion and exclusion criteria. Future studies are needed to examine the risk factors informing HCC surveillance non-compliance.

Disclosures:
Jordan L.. Redfield, MD1, Samantha A.. Whitwell, MD1, Lorri Reaves, RN2, Claudio R.. Tombazzi, MD2. P3819 - Impact of Patient Database Review on Hepatocellular Carcinoma Surveillance Rates in a Veteran Population, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Tennessee Health Science Center, Memphis, TN; 2Memphis Veterans Affairs Medical Center, Memphis, TN
Introduction: Per the American Association for the Study of Liver Diseases (AASLD), hepatocellular carcinoma (HCC) surveillance with ultrasonography (US) every six months is recommended for patients at high risk for HCC. High-risk patients include those with cirrhosis and many with chronic hepatitis B virus (HBV). HCC surveillance serves to detect HCC at an early stage, offering a more favorable prognosis. Unfortunately, many patients who qualify do not receive recommended HCC surveillance. This study sought to improve the number of patients at the Memphis Veterans Affairs Medical Center (VAMC) with cirrhosis or chronic HBV compliant with HCC surveillance.
Methods: A retrospective chart analysis of an existing patient database was performed. Three hundred patients were randomly selected from a database of 884 patients whose chart data suggested a known or possible diagnosis of cirrhosis or chronic HBV. For patients with no evidence of cirrhosis or chronic HBV upon review, charts were removed from the database. For patients with cirrhosis or chronic HBV, charts were evaluated for compliance with HCC surveillance imaging. If imaging had not been obtained within the past six months, US (or CT or MRI, as appropriate) was ordered. This chart review and intervention occurred over the course of one year; one month after completion, follow-up chart review was performed to determine if patients who were originally non-compliant with HCC surveillance were now compliant.
Results: 192 patients (64%) had cirrhosis or chronic HBV; 95 (31.7%) did not have cirrhosis or HBV; 13 (4.3%) were deceased. Of the patients with cirrhosis or chronic HBV, 111 (57.8%) were complaint with HCC surveillance, and 81 (42.2%) were non-compliant. After chart review and intervention, 139 (72.4%) were compliant with HCC surveillance, and 53 (27.6%) were non-compliant. In sum, 28 patients who were initially non-compliant with HCC surveillance were compliant upon follow-up, a 14.6% improvement.
Discussion: Database compilation and review within a large health system is an effective means of improving HCC surveillance rates in patients with cirrhosis or chronic HBV. It is likely that a longer follow-up period will demonstrate further benefit of this initiative. The large number of non-cirrhotic or non-chronic HBV patients within this database highlights the need for further refinement of inclusion and exclusion criteria. Future studies are needed to examine the risk factors informing HCC surveillance non-compliance.

Figure: Figure 1. HCC surveillance compliance before and after intervention
Disclosures:
Jordan Redfield indicated no relevant financial relationships.
Samantha Whitwell indicated no relevant financial relationships.
Lorri Reaves indicated no relevant financial relationships.
Claudio Tombazzi indicated no relevant financial relationships.
Jordan L.. Redfield, MD1, Samantha A.. Whitwell, MD1, Lorri Reaves, RN2, Claudio R.. Tombazzi, MD2. P3819 - Impact of Patient Database Review on Hepatocellular Carcinoma Surveillance Rates in a Veteran Population, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
