Tuesday Poster Session
Category: GI Bleeding
P3500 - Danger in Disguise! A Case Series of Supplement-Induced GI Bleed
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- MS
Matt Sumethasorn, MD
Keck School of Medicine of USC
Los Angeles, CA
Presenting Author(s)
Vivian H. LeTran, MD1, Matt Sumethasorn, MD1, Matthew Dukewich, MD, PharmD1, Niharika Mallepally, MD, MPH2
1Keck School of Medicine of USC, Los Angeles, CA; 2Keck School of Medicine, University of Southern California, Los Angeles, CA
Introduction: Dietary and herbal supplements (DHS) can have unlisted active ingredients, with popular brands now containing non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids. GI bleeding (GIB) caused by these supplements is clinically significant and potentially life-threatening. Unfortunately, GIB due to DHS is easily missed due to clinicians’ lack of knowledge and patient underreporting due to perceived “natural” status. We describe a case series of PUD in Hispanic patients who took a new brand of DHS.
Case Description/Methods: A 51-year-old male presented with melena and had 2 clean-based antral ulcers on EGD. He took ‘Artri King’ (AK) supplements for back pain. Further investigation found that the pills had diclofenac and dexamethasone; neither is on the product label. The patient recovered with a proton pump inhibitor (PPI). Second, a 42-year-old male who took AK Ajo pills for gout, presented with emesis. An EGD noted a 2cm duodenal ulcer (DU). The product label listed indomethacin and betamethasone. After stopping the pills, he developed adrenal insufficiency. He recovered on a steroid taper and PPI. Third, an 80-year-old male presented with melena. He took ‘garlic pills’ identified as AK. An EGD found a 4 cm DU with visible vessels. Endoscopic intervention did not achieve hemostasis and unfortunately, the patient passed away hours later.
Discussion: One-third of US adults use DHS. Per the 1994 Dietary Supplement Health and Education Act, DHS is a regulatory category that is exempt from the rigorous safety checks of traditional drug approval. Publications on DHS and GIB are limited to case reports on garlic and hawthorne. AK was developed in 2021 and not yet mentioned in literature. They are sold in Hispanic herbal stores for muscle pain, arthritis, and gout. AK products contain NSAIDs and steroids that are often not listed on the label. Our patients were Hispanic and state that AK is common in their communities. The FDA released 2 warnings this year on AK causing GIB via unlisted diclofenac and dexamethasone. Our patients were unaware, as was our team, until we thoroughly investigated medication lists. The clinical course of AK is like that of other PUD patients. Management includes transfusion, fluids, PPI, and endoscopic hemostasis. We highlight PUD due to Artri-King products, whose high-risk ingredients are usually unlisted. A detailed medication history, including specific language about dietary, herbal, or natural products- and investigation of product labels- is imperative.

Disclosures:
Vivian H. LeTran, MD1, Matt Sumethasorn, MD1, Matthew Dukewich, MD, PharmD1, Niharika Mallepally, MD, MPH2. P3500 - Danger in Disguise! A Case Series of Supplement-Induced GI Bleed, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Keck School of Medicine of USC, Los Angeles, CA; 2Keck School of Medicine, University of Southern California, Los Angeles, CA
Introduction: Dietary and herbal supplements (DHS) can have unlisted active ingredients, with popular brands now containing non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids. GI bleeding (GIB) caused by these supplements is clinically significant and potentially life-threatening. Unfortunately, GIB due to DHS is easily missed due to clinicians’ lack of knowledge and patient underreporting due to perceived “natural” status. We describe a case series of PUD in Hispanic patients who took a new brand of DHS.
Case Description/Methods: A 51-year-old male presented with melena and had 2 clean-based antral ulcers on EGD. He took ‘Artri King’ (AK) supplements for back pain. Further investigation found that the pills had diclofenac and dexamethasone; neither is on the product label. The patient recovered with a proton pump inhibitor (PPI). Second, a 42-year-old male who took AK Ajo pills for gout, presented with emesis. An EGD noted a 2cm duodenal ulcer (DU). The product label listed indomethacin and betamethasone. After stopping the pills, he developed adrenal insufficiency. He recovered on a steroid taper and PPI. Third, an 80-year-old male presented with melena. He took ‘garlic pills’ identified as AK. An EGD found a 4 cm DU with visible vessels. Endoscopic intervention did not achieve hemostasis and unfortunately, the patient passed away hours later.
Discussion: One-third of US adults use DHS. Per the 1994 Dietary Supplement Health and Education Act, DHS is a regulatory category that is exempt from the rigorous safety checks of traditional drug approval. Publications on DHS and GIB are limited to case reports on garlic and hawthorne. AK was developed in 2021 and not yet mentioned in literature. They are sold in Hispanic herbal stores for muscle pain, arthritis, and gout. AK products contain NSAIDs and steroids that are often not listed on the label. Our patients were Hispanic and state that AK is common in their communities. The FDA released 2 warnings this year on AK causing GIB via unlisted diclofenac and dexamethasone. Our patients were unaware, as was our team, until we thoroughly investigated medication lists. The clinical course of AK is like that of other PUD patients. Management includes transfusion, fluids, PPI, and endoscopic hemostasis. We highlight PUD due to Artri-King products, whose high-risk ingredients are usually unlisted. A detailed medication history, including specific language about dietary, herbal, or natural products- and investigation of product labels- is imperative.

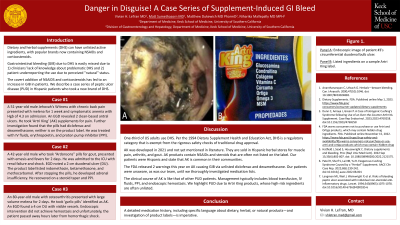
Figure: Figure 1. Panel A: Patient #3’s circumferential duodenal bulb ulcer. Panel B: Listed ingredients on a sample Artri King label.
Disclosures:
Vivian LeTran indicated no relevant financial relationships.
Matt Sumethasorn indicated no relevant financial relationships.
Matthew Dukewich: GSK – Spouse employment.
Niharika Mallepally indicated no relevant financial relationships.
Vivian H. LeTran, MD1, Matt Sumethasorn, MD1, Matthew Dukewich, MD, PharmD1, Niharika Mallepally, MD, MPH2. P3500 - Danger in Disguise! A Case Series of Supplement-Induced GI Bleed, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

