Tuesday Poster Session
Category: Esophagus
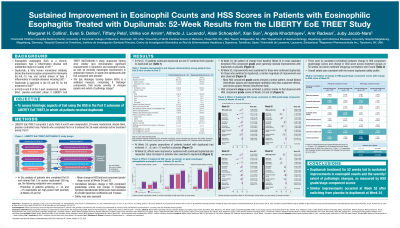
P3279 - Sustained Improvement in Eosinophil Counts and HSS Scores in Patients with Eosinophilic Esophagitis Treated with Dupilumab: 52-week Results From the LIBERTY EoE TREET Study
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Margaret H. Collins, MD
Cincinnati Children's Hospital Medical Center
Cincinnati, Ohio
Presenting Author(s)
Margaret H. Collins, MD1, Evan S. Dellon, MD, MPH2, Tiffany Pela, PharmD, MPH3, Ulrike von Arnim, MD4, Alfredo J. Lucendo, MD, PhD5, Alain Schoepfer, MD6, Xian Sun, PhD7, Angela Khodzhayev, PharmD, MS8, Amr Radwan, MBBCh, MA8, Juby A. Jacob-Nara, MD, DHSc, MPH, MBA3
1Cincinnati Children's Hospital Medical Center, Cincinnati, OH; 2University of North Carolina School of Medicine, Chapel Hill, NC; 3Sanofi, Bridgewater, NJ; 4University Hospital Magdeburg, Magdeburg, Sachsen-Anhalt, Germany; 5Hospital General de Tomelloso, Instituto de Investigación Sanitaria Princesa, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Tomelloso, Castilla-La Mancha, Spain; 6Université de Lausanne, Lausanne, Vaud, Switzerland; 7Regeneron Pharmaceuticals Inc., Bridgewater, NJ; 8Regeneron Pharmaceuticals Inc., Tarrytown, NY
Introduction: Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease associated with significant esophageal pathology. Dupilumab is approved for EoE treatment based on the 3-part, double-blind, placebo-controlled, phase 3 LIBERTY-EoE-TREET study (NCT03633617). Weekly (qw) dupilumab 300 mg demonstrated improvements vs placebo in histologic, symptomatic, and endoscopic features of EoE in Parts A and B. The EoE histology scoring system (HSS) is a validated measure, comprised of 8 histologic components, that scores severity of changes (grade) and extent of pathology (stage). Here we assess histologic aspects of EoE in the Part C extension, in which all patients (pts) received dupilumab.
Methods: Pts in Part B were randomized to dupilumab 300 mg qw, dupilumab 300 mg q2w, or placebo qw for 24 weeks (W). The current analysis includes pts who entered the extension after completing Part B and receiving dupilumab 300 mg qw.
Results: In the extension, 74 pts continued dupilumab qw and 37 switched from placebo to dupilumab qw. At extension onset (W24) higher proportions of pts treated with dupilumab than placebo had achieved eosinophil counts ≤1, ≤6, and < 15 per high-powered field (Table). During the extension effects were sustained with continued dupilumab, and responder rates increased at W52 in pts switched to dupilumab (Table). At extension onset change in basal zone hyperplasia (BZH) HSS mean (SD) grade score from baseline of the placebo-controlled part of the study had improved with dupilumab vs placebo (–2.052 [0.732] vs –0.471 [1.132]). Continued dupilumab sustained BZH improvements to W52 (–2.132 [0.744]), and placebo pts who switched at W24 improved at W52 (–2.176 [0.583]). Most HSS component grade scores showed a similar pattern (Table), except dilated intercellular spaces and dyskeratotic epithelial cells (less sustained effects), and lamina propria fibrosis (insufficient data). HSS component mean stage scores were similar. There were no consistent correlations between change in HSS component grade/stage scores and change in DSQ score across treatment groups at 52 weeks, but some moderate intragroup correlations were found. Dupilumab qw was well tolerated.
Discussion: Dupilumab treatment for 52W led to sustained improvements in eosinophil counts and the severity/extent of pathologic changes as measured by HSS grade/stage component scores. Similar improvements occurred after switching from placebo to dupilumab at W24.
Disclosures:
Margaret H. Collins, MD1, Evan S. Dellon, MD, MPH2, Tiffany Pela, PharmD, MPH3, Ulrike von Arnim, MD4, Alfredo J. Lucendo, MD, PhD5, Alain Schoepfer, MD6, Xian Sun, PhD7, Angela Khodzhayev, PharmD, MS8, Amr Radwan, MBBCh, MA8, Juby A. Jacob-Nara, MD, DHSc, MPH, MBA3. P3279 - Sustained Improvement in Eosinophil Counts and HSS Scores in Patients with Eosinophilic Esophagitis Treated with Dupilumab: 52-week Results From the LIBERTY EoE TREET Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Cincinnati Children's Hospital Medical Center, Cincinnati, OH; 2University of North Carolina School of Medicine, Chapel Hill, NC; 3Sanofi, Bridgewater, NJ; 4University Hospital Magdeburg, Magdeburg, Sachsen-Anhalt, Germany; 5Hospital General de Tomelloso, Instituto de Investigación Sanitaria Princesa, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Tomelloso, Castilla-La Mancha, Spain; 6Université de Lausanne, Lausanne, Vaud, Switzerland; 7Regeneron Pharmaceuticals Inc., Bridgewater, NJ; 8Regeneron Pharmaceuticals Inc., Tarrytown, NY
Introduction: Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease associated with significant esophageal pathology. Dupilumab is approved for EoE treatment based on the 3-part, double-blind, placebo-controlled, phase 3 LIBERTY-EoE-TREET study (NCT03633617). Weekly (qw) dupilumab 300 mg demonstrated improvements vs placebo in histologic, symptomatic, and endoscopic features of EoE in Parts A and B. The EoE histology scoring system (HSS) is a validated measure, comprised of 8 histologic components, that scores severity of changes (grade) and extent of pathology (stage). Here we assess histologic aspects of EoE in the Part C extension, in which all patients (pts) received dupilumab.
Methods: Pts in Part B were randomized to dupilumab 300 mg qw, dupilumab 300 mg q2w, or placebo qw for 24 weeks (W). The current analysis includes pts who entered the extension after completing Part B and receiving dupilumab 300 mg qw.
Results: In the extension, 74 pts continued dupilumab qw and 37 switched from placebo to dupilumab qw. At extension onset (W24) higher proportions of pts treated with dupilumab than placebo had achieved eosinophil counts ≤1, ≤6, and < 15 per high-powered field (Table). During the extension effects were sustained with continued dupilumab, and responder rates increased at W52 in pts switched to dupilumab (Table). At extension onset change in basal zone hyperplasia (BZH) HSS mean (SD) grade score from baseline of the placebo-controlled part of the study had improved with dupilumab vs placebo (–2.052 [0.732] vs –0.471 [1.132]). Continued dupilumab sustained BZH improvements to W52 (–2.132 [0.744]), and placebo pts who switched at W24 improved at W52 (–2.176 [0.583]). Most HSS component grade scores showed a similar pattern (Table), except dilated intercellular spaces and dyskeratotic epithelial cells (less sustained effects), and lamina propria fibrosis (insufficient data). HSS component mean stage scores were similar. There were no consistent correlations between change in HSS component grade/stage scores and change in DSQ score across treatment groups at 52 weeks, but some moderate intragroup correlations were found. Dupilumab qw was well tolerated.
Discussion: Dupilumab treatment for 52W led to sustained improvements in eosinophil counts and the severity/extent of pathologic changes as measured by HSS grade/stage component scores. Similar improvements occurred after switching from placebo to dupilumab at W24.
Disclosures:
Margaret Collins: Allakos – Consultant. AstraZeneca – Consultant. Bristol-Myers Squibb – Consultant. EsoCap – Consultant. Regeneron – Consultant. Sanofi – Consultant. Shire – Consultant.
Evan Dellon: Abbott – Consultant. Abbvie – Consultant. Adare/Ellodi – Consultant, Grant/Research Support. Aimmune – Consultant. Akesobio – Consultant. Alfasigma – Consultant. ALK – Consultant. Allakos – Consultant, Grant/Research Support. Amgen – Consultant. Aqilion – Consultant. Arena/Pfizer – Consultant, Grant/Research Support. Aslan – Consultant. AstraZeneca – Consultant, Grant/Research Support. Avir – Consultant. Banner Pharmaceuticals – Grant/Research Support. Biorasi – Consultant. Calypso – Consultant. Celgene/Receptos/BMS – Consultant, Grant/Research Support. Celldex – Consultant. Eli Lilly – Consultant. EsoCap – Consultant. Eupraxia – Consultant. Ferring – Consultant. Gossamer Bio – Consultant. GSK – Consultant, Grant/Research Support. Holoclara – Consultant, Grant/Research Support. Invea – Consultant, Grant/Research Support. Knightpoint – Consultant. Landos – Consultant. LucidDx – Consultant. Meritage – Grant/Research Support. Miraca – Grant/Research Support. Morphic – Consultant. Nexstone Immunology – Consultant. Nutricia – Consultant, Grant/Research Support. Parexel/Calyx – Consultant. Phathom – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Revolo Biotherapeutics – Consultant, Grant/Research Support. Robarts/Alimentiv – Consultant. Salix – Consultant. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Upstream Bio – Consultant.
Tiffany Pela: Sanofi – Employee, Stock Options.
Ulrike von Arnim: AbbVie – Consultant. Amgen – Consultant. AstraZeneca – Consultant. BMS – Consultant. Dr. Falk Foundation – Consultant. EsoCap – Consultant. EUREOS – Advisory Committee/Board Member. Galapagos – Consultant. Janssen – Consultant. MSD – Consultant. Regeneron/Sanofi – Consultant. Takeda – Consultant. Vifor – Consultant.
Alfredo Lucendo: Dr. Falk Pharma – Consultant, Grant/Research Support. EsoCap – Consultant. Regeneron Pharmaceuticals Inc. – Grant/Research Support.
Alain Schoepfer: Adare Pharma Solutions – Consultant. Aptalis – Consultant, Grant/Research Support. AstraZeneca – Grant/Research Support. Dr. Falk – Consultant, Grant/Research Support. GSK – Grant/Research Support. Nestlé – Grant/Research Support. Novartis – Grant/Research Support. Receptos/BMS – Grant/Research Support. Regeneron Pharmaceuticals Inc. – Grant/Research Support.
Xian Sun: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Angela Khodzhayev: Regeneron Pharmaceuticals Inc. – Employee, Shareholders.
Amr Radwan: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Juby Jacob-Nara: Sanofi – Employee, Stock Options.
Margaret H. Collins, MD1, Evan S. Dellon, MD, MPH2, Tiffany Pela, PharmD, MPH3, Ulrike von Arnim, MD4, Alfredo J. Lucendo, MD, PhD5, Alain Schoepfer, MD6, Xian Sun, PhD7, Angela Khodzhayev, PharmD, MS8, Amr Radwan, MBBCh, MA8, Juby A. Jacob-Nara, MD, DHSc, MPH, MBA3. P3279 - Sustained Improvement in Eosinophil Counts and HSS Scores in Patients with Eosinophilic Esophagitis Treated with Dupilumab: 52-week Results From the LIBERTY EoE TREET Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
