Tuesday Poster Session
Category: Liver
P3908 - So Long, Sotorasib: Drug-Induced Liver Injury Due to KRAS Inhibitor
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Suhind Kodali, MD
Ascension Providence Hospital
Southfield, MI
Presenting Author(s)
Suhind Kodali, MD1, Raveena Kelkar, MD2, Eric M. Sieloff, MD1, Serge Sorser, MD3
1Ascension Providence Hospital, Southfield, MI; 2Cleveland Clinic Akron General Hospital, Akron, OH; 3Ascension Providence Hospital, Novi, MI
Introduction: Drug-induced liver injury (DILI) is a frequent cause of hepatotoxicity associated with various medications and supplements. Here, we present an interesting case of DILI related to Sotorasib administration.
Case Description/Methods: A 62-year-old female with a history of lung adenocarcinoma with brain metastases presented with abdominal pain and jaundice for 1 week. Prior to this, she had completed whole brain radiation and chemotherapy and was subsequently started on Sotorasib daily. The patient was found to have elevated liver enzymes four months after Sotorasib initiation. Vital signs were within normal limits on presentation and the patient was noticeably jaundiced with scleral icterus.
Laboratory studies showed total bilirubin 10.8 mg/dL, direct bilirubin 7.8 mg/dL, ALP 646 IU/L, AST 1,028 IU/L, ALT 1,292 IU/L, and INR 1.1. LFTs were normal two months prior. Abdominal ultrasound found no biliary obstruction. Magnetic resonance cholangiopancreatography noted a 1.1 cm hemangioma. Workup for autoimmune disease and hemochromatosis was negative. Testing for viral hepatitis was negative. HSV IgM was detected, however HSV PCR was undetectable.
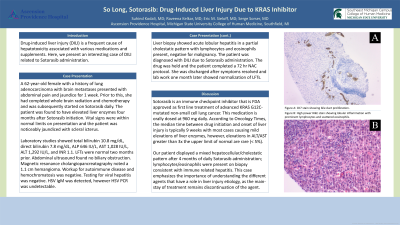
Liver biopsy showed acute lobular hepatitis in a partial cholestatic pattern with lymphocytes and eosinophils present, negative for malignancy. The patient was diagnosed with DILI due to Sotorasib administration. The drug was held and the patient completed a 72 hr NAC protocol. She was discharged after symptoms resolved and repeat lab work showed LFT improvement.
Discussion: Sotorasib is an immune checkpoint inhibitor that is FDA approved as first line treatment of advanced KRAS G12C-mutated non-small cell lung cancer. This medication is orally dosed at 960 mg daily. According to Oncology Times, the median time between drug initiation and onset of liver injury is typically 9 weeks with most cases causing mild elevations of liver enzymes, however, elevations in ALT/AST greater than 3 times the upper limit of normal are rare (< 5%). The hepatotoxicity is likely a result of a proinflammatory state, which triggers an immune related hepatitis, with mixed cholestatic and hepatocellular patterns.
Our patient displayed a mixed pattern after 4 months of daily Sotorasib administration, and lymphocytes/eosinophils were present on biopsy consistent with immune related hepatitis. This case emphasizes the importance of understanding the different agents that have a role in liver injury etiology, as the main-stay of treatment remains discontinuation of the offending agent.

Disclosures:
Suhind Kodali, MD1, Raveena Kelkar, MD2, Eric M. Sieloff, MD1, Serge Sorser, MD3. P3908 - So Long, Sotorasib: Drug-Induced Liver Injury Due to KRAS Inhibitor, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Ascension Providence Hospital, Southfield, MI; 2Cleveland Clinic Akron General Hospital, Akron, OH; 3Ascension Providence Hospital, Novi, MI
Introduction: Drug-induced liver injury (DILI) is a frequent cause of hepatotoxicity associated with various medications and supplements. Here, we present an interesting case of DILI related to Sotorasib administration.
Case Description/Methods: A 62-year-old female with a history of lung adenocarcinoma with brain metastases presented with abdominal pain and jaundice for 1 week. Prior to this, she had completed whole brain radiation and chemotherapy and was subsequently started on Sotorasib daily. The patient was found to have elevated liver enzymes four months after Sotorasib initiation. Vital signs were within normal limits on presentation and the patient was noticeably jaundiced with scleral icterus.
Laboratory studies showed total bilirubin 10.8 mg/dL, direct bilirubin 7.8 mg/dL, ALP 646 IU/L, AST 1,028 IU/L, ALT 1,292 IU/L, and INR 1.1. LFTs were normal two months prior. Abdominal ultrasound found no biliary obstruction. Magnetic resonance cholangiopancreatography noted a 1.1 cm hemangioma. Workup for autoimmune disease and hemochromatosis was negative. Testing for viral hepatitis was negative. HSV IgM was detected, however HSV PCR was undetectable.
Liver biopsy showed acute lobular hepatitis in a partial cholestatic pattern with lymphocytes and eosinophils present, negative for malignancy. The patient was diagnosed with DILI due to Sotorasib administration. The drug was held and the patient completed a 72 hr NAC protocol. She was discharged after symptoms resolved and repeat lab work showed LFT improvement.
Discussion: Sotorasib is an immune checkpoint inhibitor that is FDA approved as first line treatment of advanced KRAS G12C-mutated non-small cell lung cancer. This medication is orally dosed at 960 mg daily. According to Oncology Times, the median time between drug initiation and onset of liver injury is typically 9 weeks with most cases causing mild elevations of liver enzymes, however, elevations in ALT/AST greater than 3 times the upper limit of normal are rare (< 5%). The hepatotoxicity is likely a result of a proinflammatory state, which triggers an immune related hepatitis, with mixed cholestatic and hepatocellular patterns.
Our patient displayed a mixed pattern after 4 months of daily Sotorasib administration, and lymphocytes/eosinophils were present on biopsy consistent with immune related hepatitis. This case emphasizes the importance of understanding the different agents that have a role in liver injury etiology, as the main-stay of treatment remains discontinuation of the offending agent.

Figure: Figure A: CK7 stain showing bile duct proliferation. Figure B: H&E stain (high power) showing lobular inflammation with prominent lymphocytes and scattered eosinophils.
Disclosures:
Suhind Kodali indicated no relevant financial relationships.
Raveena Kelkar indicated no relevant financial relationships.
Eric Sieloff indicated no relevant financial relationships.
Serge Sorser indicated no relevant financial relationships.
Suhind Kodali, MD1, Raveena Kelkar, MD2, Eric M. Sieloff, MD1, Serge Sorser, MD3. P3908 - So Long, Sotorasib: Drug-Induced Liver Injury Due to KRAS Inhibitor, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
