Tuesday Poster Session
Category: IBD
P3622 - Use of Immunomodulators or Biologics and Risk of Pneumonia Among IBD Patients
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Maxwell Barffour, PhD
University of Missouri School of Medicine
Columbia, MO
Presenting Author(s)
Maxwell Barffour, PhD1, Mustafa Gandhi, MD2, Emily Reznicek, MD3, Harleen Chela, MD1, Kwame Frimpong, BSc4, Kevin Luton, BSc1, Elizabeth Karanja, BSc1, Emily Bosak, MD5, Matthew Bechtold, MD1, Yezaz Ghouri, MD6
1University of Missouri School of Medicine, Columbia, MO; 2University of Missouri-Columbia, Columbia, MO; 3University of Missouri School of Medicine, Columbia, MO; 4University of Missouri School of Medicine, Columbia, MO; 5University of Missouri School of Medicine, Columbia, MO; 6University of Missouri School of Medicine, Columbia, MO
Introduction: Inflammatory bowel disease (IBD) is an immunologic condition that is primarily treated with immunosuppressive therapy. Elevated infectious disease risk, including pneumonia, is an unintended consequence of immunosuppressive therapy. The goal of our study was to assess the risk of developing pneumonia in patients with IBD while on therapy with immunomodulators and/or biologic agents.
Methods: Data for this study was extracted from electronic health records of IBD patients seen at the University of Missouri Health System between January 2017 to April 2021. Primary outcome was pneumonia defined as a reported diagnosis of pneumonia during the period of observation. Primary exposure variables were use of immunomodulators and biologic agents. We used multivariate logistic regression models to estimate the odds of pneumonia as a function of therapeutic agent used, and controlling for age, race, gender, and use of antibiotics, corticosteroids, PPI and histamine-2 receptor antagonists during the period of observation.
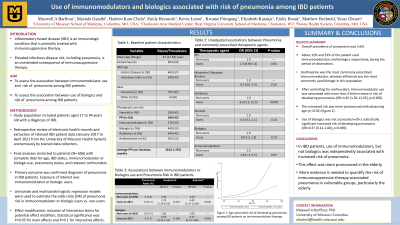
Results: The study included 606 patients diagnosed with IBD, age 17 to 94 years. Overall prevalence of pneumonia was 3.6%. About 32% and 52% of the patient used immunomodulators and biologics respectively, during the period of observation. Use of immunomodulators was associated with an elevated risk of pneumonia (table 1). After controlling for confounders, immunomodulator use was associated with more than 4-fold increase in risk of developing pneumonia (OR=4.45 [1.56-12.65], p=0.005). This increased risk was more pronounced with advancing age (p < 0.01) (figure 1). Use of biologics was not associated with a statistically significant increased risk of developing pneumonia (OR=0.37 [0.12-1.08], p=0.069).
Discussion: In IBD patients, use of immunomodulators, but not biologics was independently associated with increased incidence of pneumonia. This effect was more pronounced in the elderly. More evidence needed to quantify the risk of immunosuppressive therapy-associated pneumonia in vulnerable groups, particularly the elderly.

Disclosures:
Maxwell Barffour, PhD1, Mustafa Gandhi, MD2, Emily Reznicek, MD3, Harleen Chela, MD1, Kwame Frimpong, BSc4, Kevin Luton, BSc1, Elizabeth Karanja, BSc1, Emily Bosak, MD5, Matthew Bechtold, MD1, Yezaz Ghouri, MD6. P3622 - Use of Immunomodulators or Biologics and Risk of Pneumonia Among IBD Patients, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Missouri School of Medicine, Columbia, MO; 2University of Missouri-Columbia, Columbia, MO; 3University of Missouri School of Medicine, Columbia, MO; 4University of Missouri School of Medicine, Columbia, MO; 5University of Missouri School of Medicine, Columbia, MO; 6University of Missouri School of Medicine, Columbia, MO
Introduction: Inflammatory bowel disease (IBD) is an immunologic condition that is primarily treated with immunosuppressive therapy. Elevated infectious disease risk, including pneumonia, is an unintended consequence of immunosuppressive therapy. The goal of our study was to assess the risk of developing pneumonia in patients with IBD while on therapy with immunomodulators and/or biologic agents.
Methods: Data for this study was extracted from electronic health records of IBD patients seen at the University of Missouri Health System between January 2017 to April 2021. Primary outcome was pneumonia defined as a reported diagnosis of pneumonia during the period of observation. Primary exposure variables were use of immunomodulators and biologic agents. We used multivariate logistic regression models to estimate the odds of pneumonia as a function of therapeutic agent used, and controlling for age, race, gender, and use of antibiotics, corticosteroids, PPI and histamine-2 receptor antagonists during the period of observation.
Results: The study included 606 patients diagnosed with IBD, age 17 to 94 years. Overall prevalence of pneumonia was 3.6%. About 32% and 52% of the patient used immunomodulators and biologics respectively, during the period of observation. Use of immunomodulators was associated with an elevated risk of pneumonia (table 1). After controlling for confounders, immunomodulator use was associated with more than 4-fold increase in risk of developing pneumonia (OR=4.45 [1.56-12.65], p=0.005). This increased risk was more pronounced with advancing age (p < 0.01) (figure 1). Use of biologics was not associated with a statistically significant increased risk of developing pneumonia (OR=0.37 [0.12-1.08], p=0.069).
Discussion: In IBD patients, use of immunomodulators, but not biologics was independently associated with increased incidence of pneumonia. This effect was more pronounced in the elderly. More evidence needed to quantify the risk of immunosuppressive therapy-associated pneumonia in vulnerable groups, particularly the elderly.

Figure: Figure 1: Age associated risk of developing pneumonia among IBD patients on immunomodulator therapy.
Disclosures:
Maxwell Barffour indicated no relevant financial relationships.
Mustafa Gandhi indicated no relevant financial relationships.
Emily Reznicek indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Kwame Frimpong indicated no relevant financial relationships.
Kevin Luton indicated no relevant financial relationships.
Elizabeth Karanja indicated no relevant financial relationships.
Emily Bosak indicated no relevant financial relationships.
Matthew Bechtold indicated no relevant financial relationships.
Yezaz Ghouri indicated no relevant financial relationships.
Maxwell Barffour, PhD1, Mustafa Gandhi, MD2, Emily Reznicek, MD3, Harleen Chela, MD1, Kwame Frimpong, BSc4, Kevin Luton, BSc1, Elizabeth Karanja, BSc1, Emily Bosak, MD5, Matthew Bechtold, MD1, Yezaz Ghouri, MD6. P3622 - Use of Immunomodulators or Biologics and Risk of Pneumonia Among IBD Patients, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
