Tuesday Poster Session
Category: IBD
P3629 - Prior Anti-TNF-Alpha Exposure Is Associated with Ustekinumab Dose Escalation in Patients with Inflammatory Bowel Disease
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- NT
Nancy Tran, BA
Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine
New York, New York
Presenting Author(s)
Nancy Tran, BA1, Alexa Lavergne, BS1, Ellen Scherl, MD, FACG2, Randy Longman, MD, PhD3, Dana Lukin, MD, PhD, FACG2
1Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY; 2Jill Roberts Center for Inflammatory Bowel Disease, New York, NY; 3Weill Cornell Medical College, New York, NY
Introduction: Ustekinumab (UST) is an effective biologic therapy for treating inflammatory bowel disease (IBD). Dose escalation is frequently required for maintenance of clinical response in routine practice, but the impact of previous biologic exposure on the need for dose escalation is not well defined.1 Here, we performed a retrospective analysis of UST maintenance dosing in IBD patients at 1-year post-therapy initiation, stratified by previous anti-TNF-⍺ exposure.
Methods: Subjects were recruited from a longitudinal, single-center observational study of advanced therapy initiation in IBD patients. 137 patients were retrospectively characterized by maintenance dosing following 1 year of therapy and analyzed according to history of anti-TNF-⍺ therapy exposure. Standard maintenance dosing for UST was defined by every 8 weeks, while escalated maintenance dosing was defined by every 4 or 6 weeks. Disease activity scores, partial Mayo (pMayo) for ulcerative colitis (UC) and Harvey Bradshaw Index (HBI) for Crohn’s disease (CD), were prospectively collected 1 year after therapy initiation for patients with initially active disease (pMayo ≥ 2 or HBI ≥ 5). UC and CD clinical response were defined by pMayo reduction of ≥ 30% with rectal bleeding score ≤ 1 and HBI reduction ≥ 3, respectively.
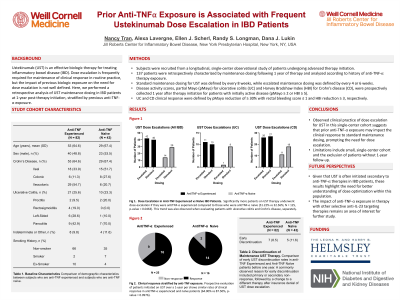
Results: Overall clinical response at 1-year did not differ according to prior anti-TNF-⍺ exposure (64.00% exposed vs. 87.50% naïve; n = 41, p-value = 0.0976). Additionally, prior anti-TNF-⍺ exposure was not associated with early UST discontinuation. Of 137 patients recruited, 12 stopped UST prior to 1-year follow-up (58.44% exposed vs. 41.67% naïve). Fifty six (44.8%) patients received q4 or q6 week dose escalation. Of these, significantly more patients with prior anti-TNF-⍺ exposure underwent UST dose escalation than those without prior exposure (51.22% vs. 32.56%; n = 125, p-value = 0.0463).
Discussion: Observed clinical practice of dose escalation for UST in this single-center cohort suggests that prior anti-TNF-⍺ exposure may impact the clinical response to standard maintenance dosing, prompting the need for dose escalation. Limitations include the limited single-center cohort and the exclusion of patients without 1-year follow-up. Given that UST is often trialed secondary to anti-TNF-⍺ therapies in IBD patients, these results highlight the need for better understanding of dose optimization within this population.
Disclosures:
Nancy Tran, BA1, Alexa Lavergne, BS1, Ellen Scherl, MD, FACG2, Randy Longman, MD, PhD3, Dana Lukin, MD, PhD, FACG2. P3629 - Prior Anti-TNF-Alpha Exposure Is Associated with Ustekinumab Dose Escalation in Patients with Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY; 2Jill Roberts Center for Inflammatory Bowel Disease, New York, NY; 3Weill Cornell Medical College, New York, NY
Introduction: Ustekinumab (UST) is an effective biologic therapy for treating inflammatory bowel disease (IBD). Dose escalation is frequently required for maintenance of clinical response in routine practice, but the impact of previous biologic exposure on the need for dose escalation is not well defined.1 Here, we performed a retrospective analysis of UST maintenance dosing in IBD patients at 1-year post-therapy initiation, stratified by previous anti-TNF-⍺ exposure.
Methods: Subjects were recruited from a longitudinal, single-center observational study of advanced therapy initiation in IBD patients. 137 patients were retrospectively characterized by maintenance dosing following 1 year of therapy and analyzed according to history of anti-TNF-⍺ therapy exposure. Standard maintenance dosing for UST was defined by every 8 weeks, while escalated maintenance dosing was defined by every 4 or 6 weeks. Disease activity scores, partial Mayo (pMayo) for ulcerative colitis (UC) and Harvey Bradshaw Index (HBI) for Crohn’s disease (CD), were prospectively collected 1 year after therapy initiation for patients with initially active disease (pMayo ≥ 2 or HBI ≥ 5). UC and CD clinical response were defined by pMayo reduction of ≥ 30% with rectal bleeding score ≤ 1 and HBI reduction ≥ 3, respectively.
Results: Overall clinical response at 1-year did not differ according to prior anti-TNF-⍺ exposure (64.00% exposed vs. 87.50% naïve; n = 41, p-value = 0.0976). Additionally, prior anti-TNF-⍺ exposure was not associated with early UST discontinuation. Of 137 patients recruited, 12 stopped UST prior to 1-year follow-up (58.44% exposed vs. 41.67% naïve). Fifty six (44.8%) patients received q4 or q6 week dose escalation. Of these, significantly more patients with prior anti-TNF-⍺ exposure underwent UST dose escalation than those without prior exposure (51.22% vs. 32.56%; n = 125, p-value = 0.0463).
Discussion: Observed clinical practice of dose escalation for UST in this single-center cohort suggests that prior anti-TNF-⍺ exposure may impact the clinical response to standard maintenance dosing, prompting the need for dose escalation. Limitations include the limited single-center cohort and the exclusion of patients without 1-year follow-up. Given that UST is often trialed secondary to anti-TNF-⍺ therapies in IBD patients, these results highlight the need for better understanding of dose optimization within this population.
Disclosures:
Nancy Tran indicated no relevant financial relationships.
Alexa Lavergne indicated no relevant financial relationships.
Ellen Scherl: AbbVie – Consultant, Grant/Research Support. AstraZeneca – Grant/Research Support. Bristol Myers Squibb – Consultant. Celgen. R.B. – Grant/Research Support. Crohn’s and Colitis Foundation of America (CCFA) – Consultant, Grant/Research Support. Entera Health – Consultant. Evidera – Consultant, Grant/Research Support. Genentech – Grant/Research Support. GI Health Foundation – Consultant. Janssen – Consultant, Grant/Research Support. Johns Hopkins University – Grant/Research Support. National Institute of Diabetes and Digestive and Kidney (NIDDK) – Grant/Research Support. National Institute of Health (NIH) – Grant/Research Support. New York Crohn’s Foundation – Grant/Research Support. Pfizer – Grant/Research Support. Prometheus – Consultant. Protagonist Therapeutics – Consultant. Seres Health – Consultant. Seres Therapeutics – Grant/Research Support. Takeda Pharmaceuticals – Consultant. UCB – Grant/Research Support. UCSF–CCFA Clinical Research Alliance – Grant/Research Support.
Randy Longman: enzymetrics – Consultant. Pfizer – Consultant.
Dana Lukin: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Consultant. Fresenius Kabi – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Magellan Health – Consultant. Palatin – Consultant. Pfizer – Consultant. Prometheus – Consultant. PSI – Consultant. Takeda – Consultant, Grant/Research Support.
Nancy Tran, BA1, Alexa Lavergne, BS1, Ellen Scherl, MD, FACG2, Randy Longman, MD, PhD3, Dana Lukin, MD, PhD, FACG2. P3629 - Prior Anti-TNF-Alpha Exposure Is Associated with Ustekinumab Dose Escalation in Patients with Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
