Tuesday Poster Session
Category: Colon
P3003 - A Phase 2 Evaluation of a New Flavored PEG and Sulfate Solution
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Jack A. Di Palma, MD
University of South Alabama
Mobile, AL
Presenting Author(s)
Peter Winkle, MD, FACG1, Gregory J. Wiener, MD, FACG2, John D. McGowan, MPH3, Jack A. Di Palma, MD4
1Cenexel, Anaheim, CA; 2GW Research, Inc., Chula Vista, CA; 3Braintree Laboratories, Inc., Braintree, MA; 4University of South Alabama, Mobile, AL
Introduction: This study describes a Phase 2 evaluation of a novel flavored PEG and sulfate solution (FPSS, also known as BLI4900) developed as a preparation for colonoscopy. FPSS was formulated to provide effective and safe cleansing but optimized for electrolyte balance and taste. FPSS features a sports drink-like taste similar to an unapproved OTC PEG and sports-drink combination (PEG-SD).
Methods: This multi-center, non-randomized study evaluated sequential cohorts of developmental formulations of FPSS in colonoscopy patients. FPSS and a PEG-SD control were given in split-dose (PM/AM) regimens starting the day before colonoscopy. Each FPSS dose consisted of 1 L of prep solution with 16 oz of additional water. PEG-SD was given to 19 patients (mean age 49) as 2 doses of PEG3350 (119 g) mixed with 32 oz of sports drink. The first PEG-SD dose was preceded by 10mg of bisacodyl at 3:00PM. 40 adults (mean age 58) were enrolled and prepped with the to-be-marketed FPSS formulation.
Since the performing endoscopists were unblinded, blinded central reading was also performed using video recordings. Cleansing was rated with an FDA-accepted 4-point global scale (Excellent, Good, Fair, Poor), with Good or Excellent considered a Success. Colon segments were also graded on withdrawal. Lab testing and adverse events (AEs) were collected for safety. Patient prep experience was assessed via questionnaire.
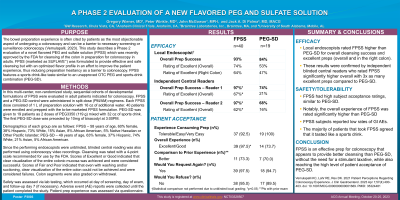
Results: A high rate of cleansing success was seen with FPSS, with 93% of preps rated as successful by performing endoscopists. Segmental success was also high (> 95%), including the right colon. PEG-SD had an overall success rate of 84%, with 89% success in the distal and transverse colon but only 69% success in the right colon. Blinded central reviewers evaluated both preps and rated the cleansing success of FPSS significantly higher than PEG-SD (Rater 1: FPSS = 97%, PEG-SD = 74%; Rater 2: FPSS = 97%, PEG-SD = 68%).
FPSS resulted in low rates of expected bowel prep symptoms. Patient acceptance scores (Table 1) were high, with 97% of FPSS patients rating their experience excellent or good versus 73.7% for PEG-SD (p< 0.05). 68% of patients agreed that FPSS tasted like a sports drink.
Discussion: This Phase 2 study confirms that FPSS is an effective prep for colonoscopy with low rates of expected AEs. It appears to provide better cleansing than PEG-SD, without the need for a stimulant laxative, while also reaching the high level of patient acceptance of PEG-SD.
Disclosures:
Peter Winkle, MD, FACG1, Gregory J. Wiener, MD, FACG2, John D. McGowan, MPH3, Jack A. Di Palma, MD4. P3003 - A Phase 2 Evaluation of a New Flavored PEG and Sulfate Solution, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Cenexel, Anaheim, CA; 2GW Research, Inc., Chula Vista, CA; 3Braintree Laboratories, Inc., Braintree, MA; 4University of South Alabama, Mobile, AL
Introduction: This study describes a Phase 2 evaluation of a novel flavored PEG and sulfate solution (FPSS, also known as BLI4900) developed as a preparation for colonoscopy. FPSS was formulated to provide effective and safe cleansing but optimized for electrolyte balance and taste. FPSS features a sports drink-like taste similar to an unapproved OTC PEG and sports-drink combination (PEG-SD).
Methods: This multi-center, non-randomized study evaluated sequential cohorts of developmental formulations of FPSS in colonoscopy patients. FPSS and a PEG-SD control were given in split-dose (PM/AM) regimens starting the day before colonoscopy. Each FPSS dose consisted of 1 L of prep solution with 16 oz of additional water. PEG-SD was given to 19 patients (mean age 49) as 2 doses of PEG3350 (119 g) mixed with 32 oz of sports drink. The first PEG-SD dose was preceded by 10mg of bisacodyl at 3:00PM. 40 adults (mean age 58) were enrolled and prepped with the to-be-marketed FPSS formulation.
Since the performing endoscopists were unblinded, blinded central reading was also performed using video recordings. Cleansing was rated with an FDA-accepted 4-point global scale (Excellent, Good, Fair, Poor), with Good or Excellent considered a Success. Colon segments were also graded on withdrawal. Lab testing and adverse events (AEs) were collected for safety. Patient prep experience was assessed via questionnaire.
Results: A high rate of cleansing success was seen with FPSS, with 93% of preps rated as successful by performing endoscopists. Segmental success was also high (> 95%), including the right colon. PEG-SD had an overall success rate of 84%, with 89% success in the distal and transverse colon but only 69% success in the right colon. Blinded central reviewers evaluated both preps and rated the cleansing success of FPSS significantly higher than PEG-SD (Rater 1: FPSS = 97%, PEG-SD = 74%; Rater 2: FPSS = 97%, PEG-SD = 68%).
FPSS resulted in low rates of expected bowel prep symptoms. Patient acceptance scores (Table 1) were high, with 97% of FPSS patients rating their experience excellent or good versus 73.7% for PEG-SD (p< 0.05). 68% of patients agreed that FPSS tasted like a sports drink.
Discussion: This Phase 2 study confirms that FPSS is an effective prep for colonoscopy with low rates of expected AEs. It appears to provide better cleansing than PEG-SD, without the need for a stimulant laxative, while also reaching the high level of patient acceptance of PEG-SD.
Disclosures:
Peter Winkle indicated no relevant financial relationships.
Gregory Wiener indicated no relevant financial relationships.
John McGowan: Braintree Laboratories, Inc. – Employee.
Jack Di Palma: Sebela – Consultant.
Peter Winkle, MD, FACG1, Gregory J. Wiener, MD, FACG2, John D. McGowan, MPH3, Jack A. Di Palma, MD4. P3003 - A Phase 2 Evaluation of a New Flavored PEG and Sulfate Solution, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

