Tuesday Poster Session
Category: Colon
P3009 - Gastrointestinal Adverse Events Following Brentuximab Vedotin and Polatuzumab Vedotin Therapy
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- AK
Andrew Kuang, MD
Baylor College of Medicine
New York, NY
Presenting Author(s)
Andrew Kuang, MD1, Jay S. Shah, MD1, Nitish Mittal, MD2, Malek Shatila, MD3, Sidra Naz, MD, MPH3, Swaminathan Iyer, MD3, Anusha Thomas, MD3, Hao Chi Zhang, MD3, Yinghong Wang, MD3
1Baylor College of Medicine, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Brentuximab vedotin (BV), a CD30-specific monoclonal antibody conjugate, and polatuzumab vedotin (PV), a CD79b-specific monoclonal antibody conjugate, are targeted therapies used in the treatment of hematologic cancers. Both have been observed to cause gastrointestinal adverse events (GI AE). We studied the characteristics and management of these GI AE related to BV and PV therapy.
Methods: 879 adult cancer patients were retrospectively identified who received either BV or PV therapy between 3/2016 and 3/2023 at our tertiary cancer center. Patients with alternate diagnoses, such as GI infections or history of chronic GI diseases were excluded. Clinical characteristics, management, and outcomes were evaluated.
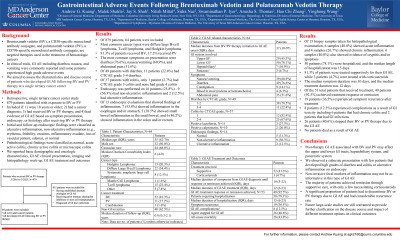
Results: 64 patients (7.3%) were eligible and included. The median patient age was 55 years. The median duration from BV or PV initiation to GI AE onset was 37 days. The distribution of toxicity ranged from lower GI (78.1%), upper GI (45.3%), hepatobiliary (10.9%), and pancreatic (4.3%). Common symptoms were diarrhea (76.6%), nausea (60.9%), and abdominal pain (51.6%). Few patients had CTCAE grade ≥3 GI toxicity (19.4% with diarrhea and 2.7% with colitis symptoms). 25.8% of patients received endoscopic evaluation with inflammation seen in esophagus/stomach (43.8%), small intestine (31.3%), and colon/rectum (37.5%). 12.5% of patients had ulcerative inflammation. Of those biopsied, 40.0% showed acute inflammation and 26.7% showed chronic inflammation. The median symptom duration was 10 days. 81.3% of patients were treated supportively, while 3 patients received corticosteroids. The median duration of all treatments was 12 days and 92.5% of patients had symptom resolution following treatment. Symptom recurrence occurred in 36.5% of patients and 8 patients had GI AE-related complications including chronic colitis (9.4%) and GI infection (3.1%). 40.6% of patients stopped BV or PV therapy due to GI AE.
Discussion: Targeted antibody-drug conjugates may be related to post-therapy GI toxicity that can involve the upper and lower GI tracts, hepatobiliary, and pancreatic systems. We observed a subacute presentation with few patients that developed high grades of diarrhea and colitis or ulcerative inflammation on endoscopy. Most patients achieved remission with supportive management with few requiring corticosteroids. Despite this, a large number of patients stopped BV or PV therapy due to GI AE. Future large-scale studies may provide clarification.
Disclosures:
Andrew Kuang, MD1, Jay S. Shah, MD1, Nitish Mittal, MD2, Malek Shatila, MD3, Sidra Naz, MD, MPH3, Swaminathan Iyer, MD3, Anusha Thomas, MD3, Hao Chi Zhang, MD3, Yinghong Wang, MD3. P3009 - Gastrointestinal Adverse Events Following Brentuximab Vedotin and Polatuzumab Vedotin Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Baylor College of Medicine, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Brentuximab vedotin (BV), a CD30-specific monoclonal antibody conjugate, and polatuzumab vedotin (PV), a CD79b-specific monoclonal antibody conjugate, are targeted therapies used in the treatment of hematologic cancers. Both have been observed to cause gastrointestinal adverse events (GI AE). We studied the characteristics and management of these GI AE related to BV and PV therapy.
Methods: 879 adult cancer patients were retrospectively identified who received either BV or PV therapy between 3/2016 and 3/2023 at our tertiary cancer center. Patients with alternate diagnoses, such as GI infections or history of chronic GI diseases were excluded. Clinical characteristics, management, and outcomes were evaluated.
Results: 64 patients (7.3%) were eligible and included. The median patient age was 55 years. The median duration from BV or PV initiation to GI AE onset was 37 days. The distribution of toxicity ranged from lower GI (78.1%), upper GI (45.3%), hepatobiliary (10.9%), and pancreatic (4.3%). Common symptoms were diarrhea (76.6%), nausea (60.9%), and abdominal pain (51.6%). Few patients had CTCAE grade ≥3 GI toxicity (19.4% with diarrhea and 2.7% with colitis symptoms). 25.8% of patients received endoscopic evaluation with inflammation seen in esophagus/stomach (43.8%), small intestine (31.3%), and colon/rectum (37.5%). 12.5% of patients had ulcerative inflammation. Of those biopsied, 40.0% showed acute inflammation and 26.7% showed chronic inflammation. The median symptom duration was 10 days. 81.3% of patients were treated supportively, while 3 patients received corticosteroids. The median duration of all treatments was 12 days and 92.5% of patients had symptom resolution following treatment. Symptom recurrence occurred in 36.5% of patients and 8 patients had GI AE-related complications including chronic colitis (9.4%) and GI infection (3.1%). 40.6% of patients stopped BV or PV therapy due to GI AE.
Discussion: Targeted antibody-drug conjugates may be related to post-therapy GI toxicity that can involve the upper and lower GI tracts, hepatobiliary, and pancreatic systems. We observed a subacute presentation with few patients that developed high grades of diarrhea and colitis or ulcerative inflammation on endoscopy. Most patients achieved remission with supportive management with few requiring corticosteroids. Despite this, a large number of patients stopped BV or PV therapy due to GI AE. Future large-scale studies may provide clarification.
Disclosures:
Andrew Kuang indicated no relevant financial relationships.
Jay Shah indicated no relevant financial relationships.
Nitish Mittal indicated no relevant financial relationships.
Malek Shatila indicated no relevant financial relationships.
Sidra Naz indicated no relevant financial relationships.
Swaminathan Iyer indicated no relevant financial relationships.
Anusha Thomas indicated no relevant financial relationships.
Hao Chi Zhang indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Andrew Kuang, MD1, Jay S. Shah, MD1, Nitish Mittal, MD2, Malek Shatila, MD3, Sidra Naz, MD, MPH3, Swaminathan Iyer, MD3, Anusha Thomas, MD3, Hao Chi Zhang, MD3, Yinghong Wang, MD3. P3009 - Gastrointestinal Adverse Events Following Brentuximab Vedotin and Polatuzumab Vedotin Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

